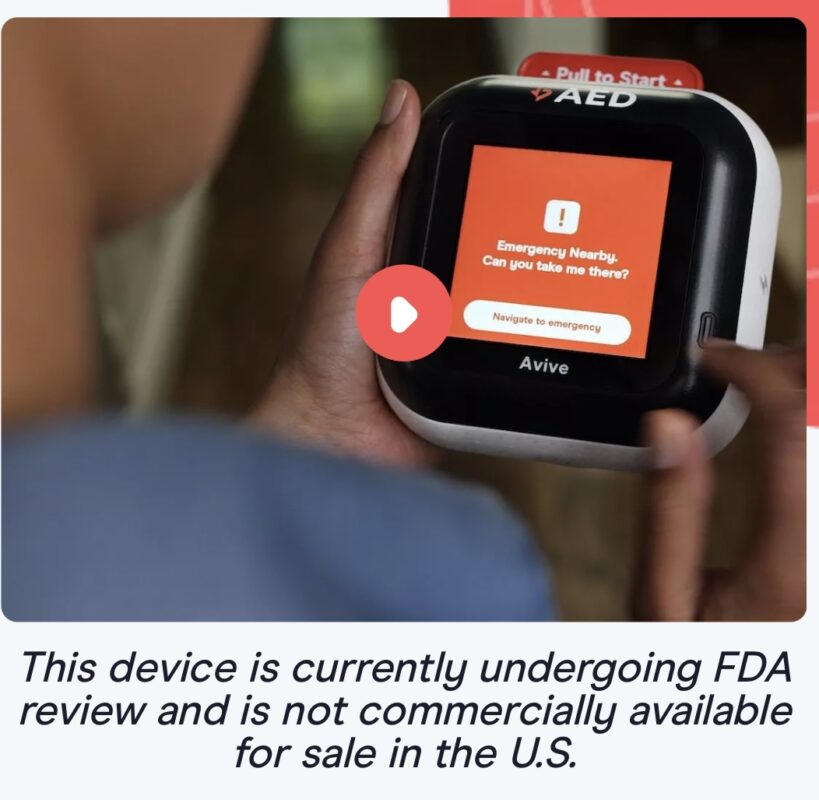
Avive Solutions, Inc, is a company bringing to market a revolutionary connected Automated External Defibrillator (AED) coupled with a first of its kind out-of-hospital cardiac arrest (OHCA) response platform. Today Avive Solutions announced a partnership with RapidSOS.
Together, the solutions are designed to quickly get life-saving AEDs to cardiac arrest patients in their greatest time of need and provide telecommunicators with unprecedented incident data to help them more seamlessly and effectively coordinate life-saving care. Built in partnership with public safety, RapidSOS’s emergency response data platform securely links life-saving data from over 350M connected devices directly to over 5,000 Emergency Communications Centers (ECCs) in North America.
Cardiac arrest is one of the leading causes of death outside the hospital globally. With a mortality rate of ~90% in the United States, OHCA leads to over 350,000 deaths per year. Fortunately, there is a proven therapy for cardiac arrest. While Cardiopulmonary Resuscitation (“CPR”) “buys time,” a person in cardiac arrest ultimately needs to receive a shock delivered from an AED to survive. However, the greatest challenge with cardiac arrest emergencies is time, as every minute a person in cardiac arrest goes without receiving a defibrillation shock reduces their chances of survival by 7-10%. Despite their best efforts, the average response time of Emergency Medical Services (EMS) in the U.S. is 7 minutes, and increasing to more than 14 minutes in more rural locations, making immediate access to an AED and use of the device by everyday people prior to EMS arrival critical for the survival chances of a person in cardiac arrest.
Unfortunately, the status quo today is that an AED is used prior to EMS arrival, only ~2% of the time. This is largely because the locations of AEDs are unknown, and more importantly, because everyday people who may happen to be close to an AED when there is a cardiac arrest emergency nearby, don’t know that device could be used to save a life of someone just minutes away. These examples of critical data gaps pose a great challenge for 911 telecommunicators, who are arguably the most important first responder for cardiac arrest emergencies, as they are tasked with coordinating care with the caller on scene in the earliest, and most crucial, minutes of a cardiac arrest emergency.
Avive Solutions and RapidSOS are committed to developing innovative solutions that aim to take a new approach at improving the status quo of OHCA response. With this partnership, Emergency Communication Centers (ECCs) with access to RapidSOS will be able to dispatch Avive’s intelligent AEDs to the location of a cardiac arrest emergency. Once dispatched, Avive’s AEDs nearby the incident will audibly alert and display a map to navigate willing everyday people to the location of the person in cardiac arrest, creating the potential to greatly increase the likelihood of AEDs being used before EMS arrives on scene.
Beyond dispatching Avive’s AEDs to cardiac arrest emergencies, 911 telecommunicators will also be able to use RapidSOS Portal during the emergency to view incident data from Avive’s AEDs. This incident data includes the location of AEDs as they are en route to the emergency, data from the AED while it’s being used – which is designed to provide 911 dispatchers with insights on if and how they can help a person use the device more quickly and effectively – and much more.
“Every day ~1,000 people of all ages, races, and genders die from cardiac arrest outside of the hospital in the U.S. alone, and tragically survival rates have not appreciably improved over the last two decades,” said Sameer Jafri, President of Avive Solutions, Inc. “We are excited to partner with an innovator like RapidSOS to completely rethink the delivery of life-saving therapy to people suffering cardiac arrest in their greatest time of need.”
According to Michael Martin, CEO of RapidSOS, “We’re united in our commitment to empower safer, stronger communities with intelligent, data-driven emergency response worldwide. Together with Avive, we’re giving telecommunicators the power to activate an added layer of protection during cardiac arrests so that communities can help first responders save more lives.”
While Avive’s AED is in the FDA review cycle, and has not yet been approved for sale in the U.S., cities and counties have already expressed great interest in adopting this solution when it’s available to help improve cardiac arrest response in their community via Avive’s 4-Minute City Program.