A biomechanical evaluation of the novel LinQ device revealed statistically significant fixation of the sacroiliac (SI) joint. Developed by Tampa-based medical device company, PainTEQ, the LinQ SI Joint Stabilization System is a minimally invasive procedure in which a surgeon inserts a small, single patented implant into the SI joint. The results of this study provided validation for the novel system, showing that LinQ reduces range of motion (ROM) in all motion planes of the SI joint, providing an ideal environment for long-term fusion.
“We are thrilled to share the work of our investigators on this landmark biomechanical study on the direct fixation effects of LinQ,” said Dawood Sayed M.D., Professor of Anesthesiology and Pain Medicine at The University of Kansas Medical Center and one of the study’s key investigators. “High quality biomechanical publications are critical for validating these surgical techniques across the interventional community.”
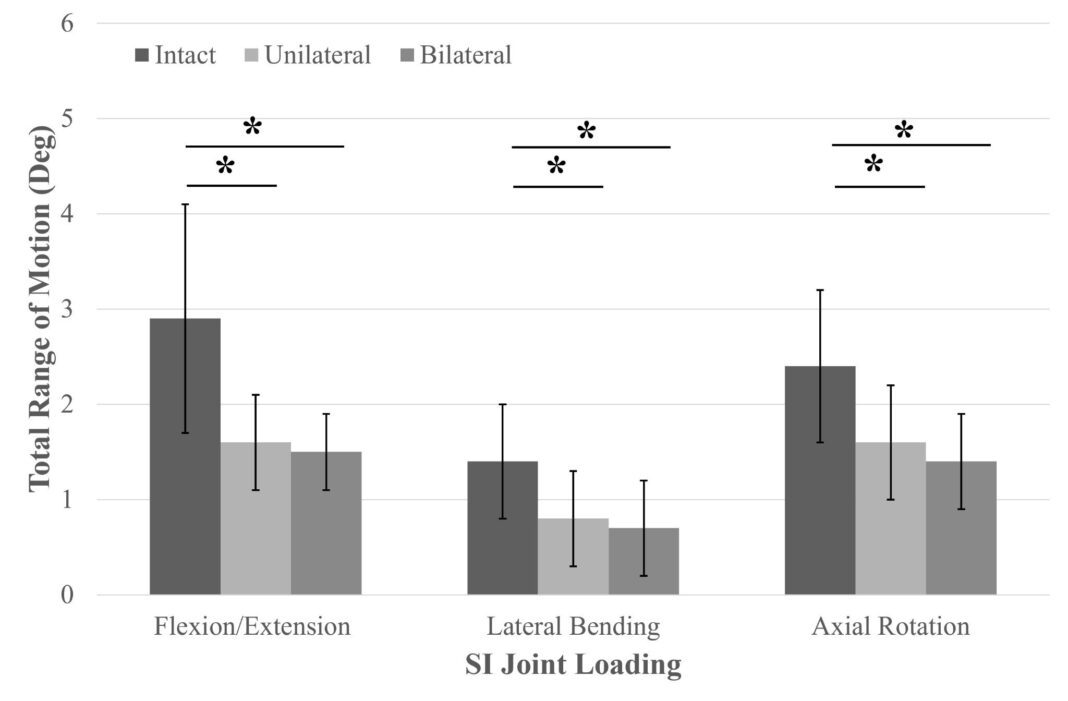
Natural degenerative changes of the SI joint can lead to chronic low back pain as movement in the joint impinges on surrounding nerves. This biomechanical evaluation studied the effectiveness of LinQ in reducing the SI joint’s ROM, which in turn, would reduce pressure on surrounding nerves.
“As a physician, we have several products and techniques available for sacroiliac fusion, but LinQ currently stands alone in having superior biomechanical outcomes backed by a high-quality peer-reviewed publication to support its use,” Dr. Sayed said.
During their research, investigators measured the total ROM using an optical tracking system before and after surgically implanting LinQ. The results revealed that LinQ significantly reduced ROM in flexion/extension, axial rotations, and lateral bends, resulting in increased multiplanar stability. Additionally, LinQ shifted the center of rotation to the implant’s location, ensuring little to no micromotion at the implantation site.
“The biomechanical stability of the LinQ procedure has demonstrated comparable results to the published lateral approach evidence,” said Kasra Amirdelfan, M.D., another one of the study’s key investigators. “The results of this study underscore LinQ’s posterior approach as a potential new standard for patients due to its minimally invasive nature and more tolerable post-operative period.”
Dr. Amirdelfan goes on to say, “The biomechanical evidence, the upcoming prospective study results, and our own clinical experience clearly show PainTEQ’s commitment to improving patient outcomes through evidence-based medicine.”
Delivering on their promise of high-quality clinical data, PainTEQ plans to release a comparative biomechanical analysis in the near future to showcase the benefits of a posterior approach to SI joint fusion over the traditional lateral technique.
“Thank you to our physician investigators for their commitment to producing these important publications,” said Shanth Thiyagalingam, Chief Commercial Officer at PainTEQ. “With our continued dedication to clinical data, the compelling body of evidence for the LinQ system continues to grow. This ultimately allows more patients to benefit from the outstanding outcomes of the LinQ system achieved through its minimally invasive, patient-centric approach.”
Those interested in being the first to know about PainTEQ’s upcoming studies can follow the company on LinkedIn: www.linkedin.com/company/painteq/.
About PainTEQ: PainTEQ was built to bring interventional procedures to the market. Working with pain management specialists to help reduce and eliminate SI joint dysfunction, PainTEQ’s LinQ therapy aims to immediately provide clinical benefits to individuals living with incapacitating lower back pain through a minimally invasive outpatient procedure.
About LinQ: The LinQ SI Joint Stabilization System provides patients with a minimally invasive option to combat pain. After a thorough diagnostic process, physicians may help alleviate, and in many cases eliminate, chronic pain by placing a single LinQ allograft into the SI Joint. With its large graft window, this single implant helps create an ideal environment for long-term fusion.