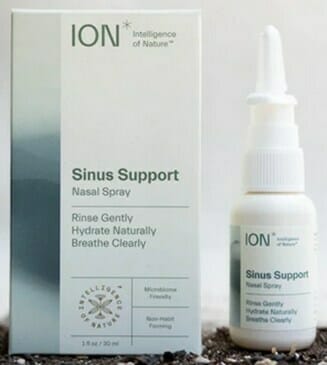
Biomic Sciences is voluntarily recalling all lots of ION* Sinus Support, ION* Biome Sinus, and Restore Sinus Spray products to the consumer level. FDA testing found the product to contain microbial contamination identified as Microbacterium sp., Fictibacillus sp., Bacillus sp., (primarily B. malikii), and Paenibacillus sp (these microorganisms are generally non-pathogenic).
Risk Statement: In the population most at risk, patients or individuals who recently underwent nasal or sinus surgery, there is a reasonable probability that the use of the affected product could potentially result in severe or life-threatening adverse events such as bacteremia or fungemia, invasive bacterial or fungal rhinosinusitis, or disseminated fungal infection. To date, Biomic Sciences has not received any reports of adverse events related to this product.
The product is used as a nasal rinse and is packaged in individual boxes of one or two nasal spray dispensers (see the product pictures below). All lots of the of the following products are covered by this recall:
| Product Name | Sales Period |
| ION* Sinus Support | September 2021 through September 20, 2023 |
| ION* Biome Sinus | September 2019 through September 2021 |
| Restore Sinus Spray | June 2017 through September 2019 |
The affected lots were distributed to wholesale and retail outlets, as well as sold directly to consumers online.
Biomic Sciences is notifying its distributors and customers by electronic communications and is arranging for the disposition of all recalled products. Consumers and other parties that have the recalled product should stop using the product and either discard it or take a photo of the lot number on the bottom of the bottle before discarding it and provide that to Biomic Sciences to arrange a refund (contact information provided below).
Parties with questions about this recall can contact ION* Sinus Recall Team at 1-844-715-0113 Monday – Friday from 9:00 am to 5:00 pm Eastern time or sinusrecall@intelligenceofnature.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.