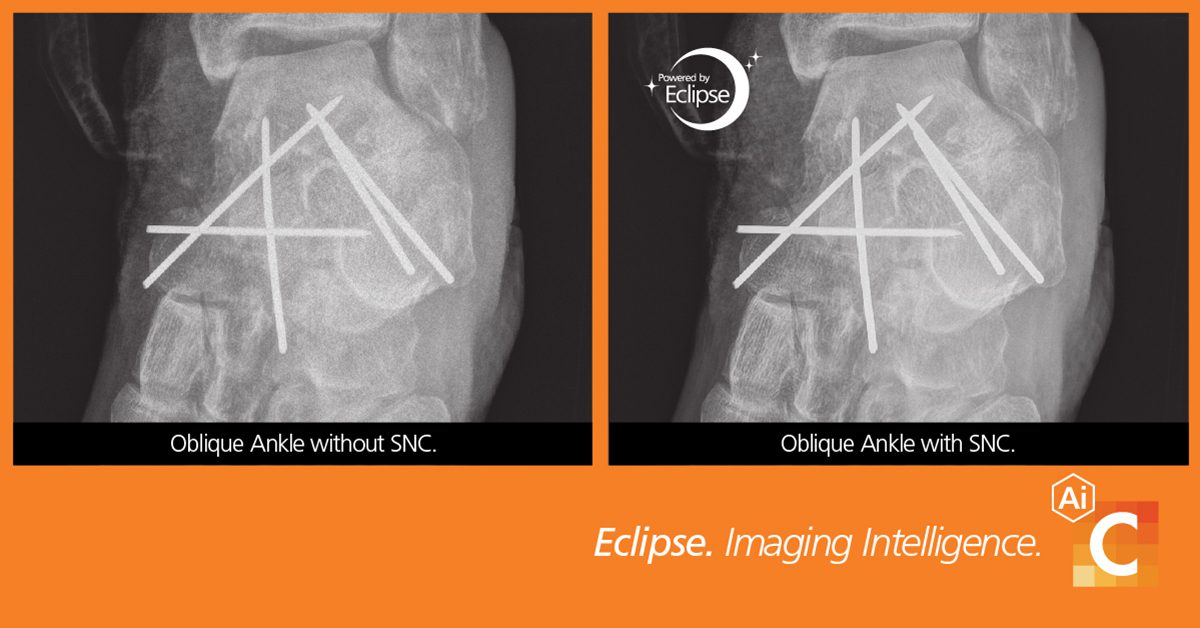
Carestream Health has released Smart Noise Cancellation (SNC), a groundbreaking artificial intelligence (AI)-based technology that greatly improves image quality—producing images that are significantly clearer than with standard processing.
SNC has received FDA 510(k) Clearance and is available as an optional feature with Carestream’s ImageView Software powered by Eclipse—the intelligent image-processing engine behind the company’s innovative imaging software—on DRX-Evolution and DRX-Evolution Plus systems.
“Carestream is a leader in using AI for noise cancellation with X-ray images. Our team of imaging scientists has been able to separate image noise from sharpness and contrast using AI-based algorithms that result in remarkable image quality,” said Jill Hamman, Worldwide Marketing Manager, Global X-ray Solutions at Carestream. “This technology provides improved anatomical clarity, preservation of fine detail and better contrast-to-noise ratio for images acquired at a broad range of exposures, which can help improve diagnostic confidence and alleviate physician fatigue. It also enables radiology professionals to better optimize radiation dose.” Optimizing radiation dose is especially important with neonatal and pediatric diagnostic imaging, where imaging at the lowest possible dose is crucial for young patients.
Separating noise from an image has been a challenge for medical imaging scientists. Traditional noise reduction introduces blurring, which degrades image sharpness and might remove important anatomical information. Conversely, the more an image is sharpened, the more noise may be enhanced. Noise is often an undesirable by-product of image capture and can obscure critical anatomical data. Carestream’s SNC is able to isolate noise to produce images that are significantly clearer than with standard processing.
As the preferred level of noise on X-ray images is subjective—for example, some radiologists expect to see a certain degree of noise in images, which assures them that the patient was not overexposed—Carestream enables imaging professionals to adjust the amount of noise cancellation and exposure to meet their desired image quality.
Carestream Health notes objective testing demonstrated that SNC processing enables a 2x–4x noise reduction in flat image areas, preserves high frequency sharpness and improves contrast detail. Additionally, a blind Clinical Reader Study using board-certified radiologists found that 89.5 percent of all study ratings showed a slight to strong preference for SNC-processed images. Sixty-four percent of the diagnostic quality ratings improved—based on the RadLex rating scale—and 56 percent of these ratings improved from “limited” or “diagnostic” to “exemplary.”
Carestream Health notes when combined with SmartGrid software, Smart Noise Cancellation software promises benefits in gridless imaging where the removal of scatter typically leads to an increase in noise appearance.
“At Carestream, improving image quality is a top priority for our R&D team,” Ms. Hamman said. “Smart Noise Cancellation provides the ability to consistently deliver high-quality diagnostic images for healthcare providers and their patients.”