LUMESEAL Laser CE Approval News
LSO MEDICAL SAS, international leader in vascular laser therapies, announced today the CE Approval of its newest generation EVLT SnakeBack® assisted system, the LUMESEAL® laser platform.
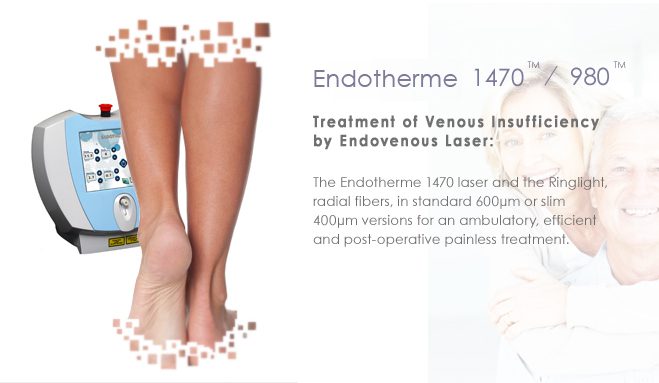
Designed to enhance ease-of-use and to provide greater precision and control throughout the procedure, Lumeseal is announced as the successor of the efficient and widely used ENDOTHERME 1470 platform.
The third-generation laser system incorporates the same 1470nm laser design combined with the RINGLIGHT® fibers range design which has shown superior performance and clinical evidence compared to other alternative configurations. In addition, LUMESEAL® incorporates the last patented innovation of the company: the SnakeBack® technology. SnakeBack® is a tensionless assisted pullback system, able to plan, apply and monitor energy profiles delivery into the vein. Based on its assisted pullback feature, and its laser driver interface, the full integration of SnakeBack® together with the BackReflection features of Endotherme 1470 allows a total supervision of the vein occlusion, while maintaining the usual touch feel feedback during procedure.
“The integration of SnakeBack® technology on LUMESEAL® is a big step forward for the development of the technique. Beyond its direct impact on enhancing the reproducibility and comfort of laser delivery, the LUMESEAL® platform allows us to assess further improvements in the EVLT technique (new wavelengths, applicators, …) “says M. Rochon, LSO Medical CEO.
Limited commercial release due to the international shortage around the semiconductor is planned for the fall with a full launch anticipated in early 2022.
For more medical device industry news, click here.