Centinel Spine®, LLC, (“the Company”) a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access, today announced the 100th completed procedure in the United States with its prodisc® C Vivo Cervical Total Disc Replacement (TDR) product.
This milestone was achieved quickly after the Company announced the first U.S. implantation of the prodisc C Vivo on Sept. 7. During this short period, the Company has trained over 70 surgeons and more than 30 new surgeons are now using the system.
According to Dr. Kevin Rutz, an Orthopedic Spine Surgeon from St. Louis, Missouri, “I have had the opportunity to use the prodisc C Vivo system multiple times and this device offers unique benefits to the surgeon and the patient. Because the Vivo implant is keel-less and incorporates a streamlined surgical technique, I have found that I am able to reduce the number of x-rays required during implantation of the device. This is a benefit to both the patient as well as the staff in the operating room. I have also found that making intra-operative adjustments to the positioning of the implant is easier with the Vivo device than other total discs that I have used in the past.”
“Degenerative disc disease is a leading cause of pain and disability in the U.S. and around the world,” said Centinel Spine CEO Steve Murray. “Providing total disc replacement solutions that can match individual patient anatomy is an important step in addressing this all-too-prevalent condition. We are pleased to see surgeons rapidly adopting the prodisc C Vivo technology knowing that many patients are benefiting from our broadened total disc portfolio.”
According to Dr. Adam Bruggeman, an Orthopedic Spine Surgeon from San Antonio, Texas, “The prodisc C Vivo system incorporates the simplicity of a keel-less disc replacement implant without sacrificing stability, an issue seen in many other implants. This is the best of both worlds in disc replacement and will rapidly be adopted by surgeons who embrace motion preservation.”
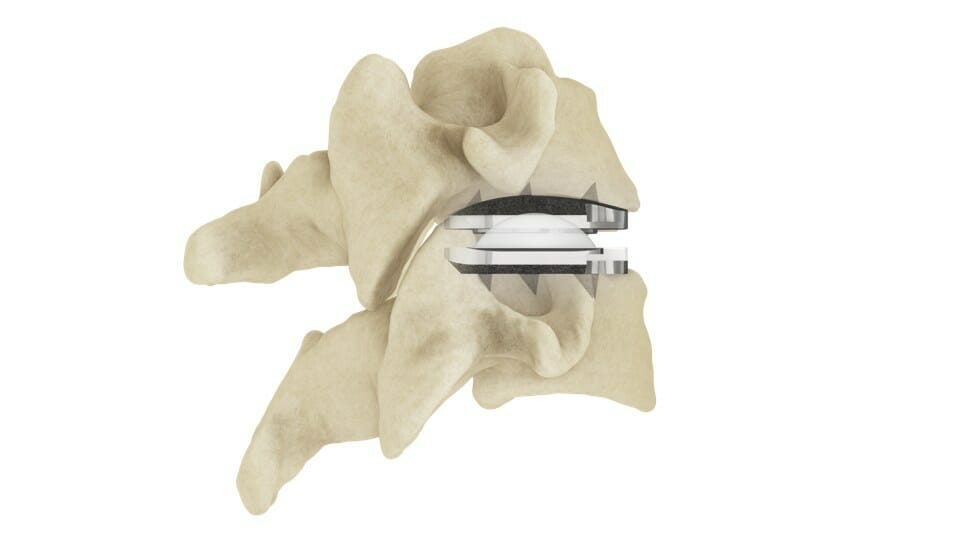
The prodisc C Vivo system has been in clinical use internationally since 2009 and is currently one of the most frequently implanted TDR devices in the world. The device has keel-less fixation and combines a unique anatomically-designed superior endplate with lateral spikes to optimize fit and provide immediate fixation. Similar to all prodisc products, the prodisc C Vivo device incorporates prodisc CORE technology, the basis behind the predictable clinical outcomes of the prodisc platform after 30 years and over 225,000 implantations worldwide.*
Centinel Spine’s cervical TDR portfolio includes four devices approved by the U.S. Food and Drug Administration for 1-level indications, offering the broadest spectrum of solutions to address surgeon preference and individual patient anatomy.