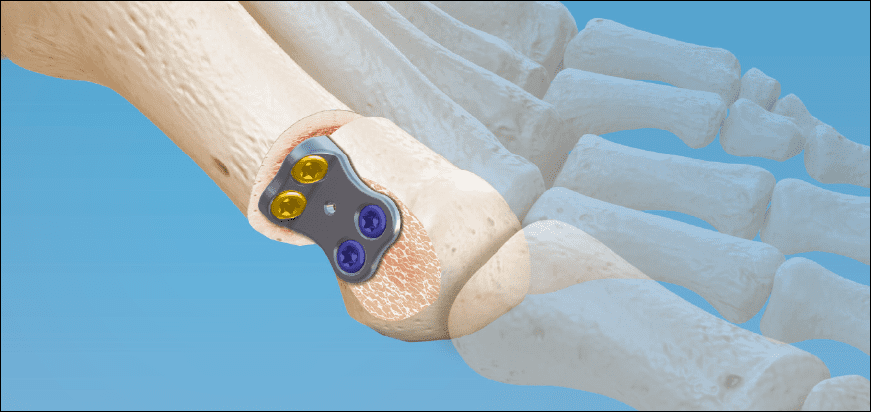
CoLink Vallux Active Bunion has been launched by In2Bones Global, Inc. It is a new, minimally invasive, joint-sparing correction technique for moderate to severe bunions.
The Active Bunion implant and guided translational osteotomy technique improve on traditional open fixation approaches as well as newer bunion surgeries by providing surgeons with a simpler, faster procedure that doesn’t violate the joint or require a 1st Tarsometatarsal joint fusion.
The implant consists of a zero-prominence CoLink Vallux™ plate and a choice of non-locking and variable angle locking screws for enhanced angular stability, together with a full instrument set.
Using this new, minimally invasive procedure, surgeons can correct joint alignment in multiple dimensions while avoiding any restriction of the joint space and not fusing the joint at the midfoot. The result is total range of motion preservation and superior cosmesis via a much smaller incision of 1-2 cm, compared with the larger incision required for Chevron osteotomy (5-7 cm).
For the patient, the Active Bunion’s smaller incision and technique as a whole reduces internal scarring compared with other bunion corrections, decreasing the typical postoperative stiffness, pain, and potential for wound complications.
“Patients prefer the smallest possible incision, and they do not want a fused joint unless absolutely necessary,” explains Jon Simon, Executvie Vice President of Commerical at In2Bones. “The CoLink Vallux Active Bunion fulfills both of these patient demands.”
An Evolved Surgery with More Surgeon and Patient Benefits
With greater simplicity comes shorter OR time. The Active Bunion procedure can typically be completed in about 20 minutes, compared to 40-60 minutes plus for other more invasive and complex corrections. Recovery can also be shorter than traditional bunion surgeries. With no joint violation, which preserves the total range of motion, patients may return to weight-bearing activity sooner, with less physical therapy.
The Active Bunion’s evolved technique, technology, and fixation solution is intended to address over 90 percent of bunion cases. Compare it to other established hallux valgus procedures:
Lapidus – Unlike this end-stage procedure, the Active Bunion’s simpler, quicker guided implant technique cuts down on multiple surgical steps, thereby decreasing OR time. It spares the midfoot joint from fusion, with early weight-bearing, improved cosmesis, and fewer overall complications.
Percutaneous Screw Fixation – Compared to the blind resection and steep learning curve required for the Burr osteotomy, the Active Bunion procedure is reproducible and straightforward. The guided osteotomy reduces the risk of shortening or necrosis.
IM Blade Plates – Eliminating multiple surgical steps common to this technique, the Active Bunion procedure allows surgeons to dictate placement while the implant provides greater strength and stability through two distal and proximal screws instead of the customary single screw.
Like all other products in In2Bones’ extensive platform of single-use implants for the hand and foot, the CoLink Vallux™ Active Bunion system is packaged sterile for traceability and patient safety.