Conformal Medical today announced it has secured $85 million in Series C financing. The financing syndicate included participation from new investors Fidelity Management & Research Company LLC and an undisclosed strategic investor, as well as Catalyst Health Ventures (CHV) and many returning Series A and B investors.
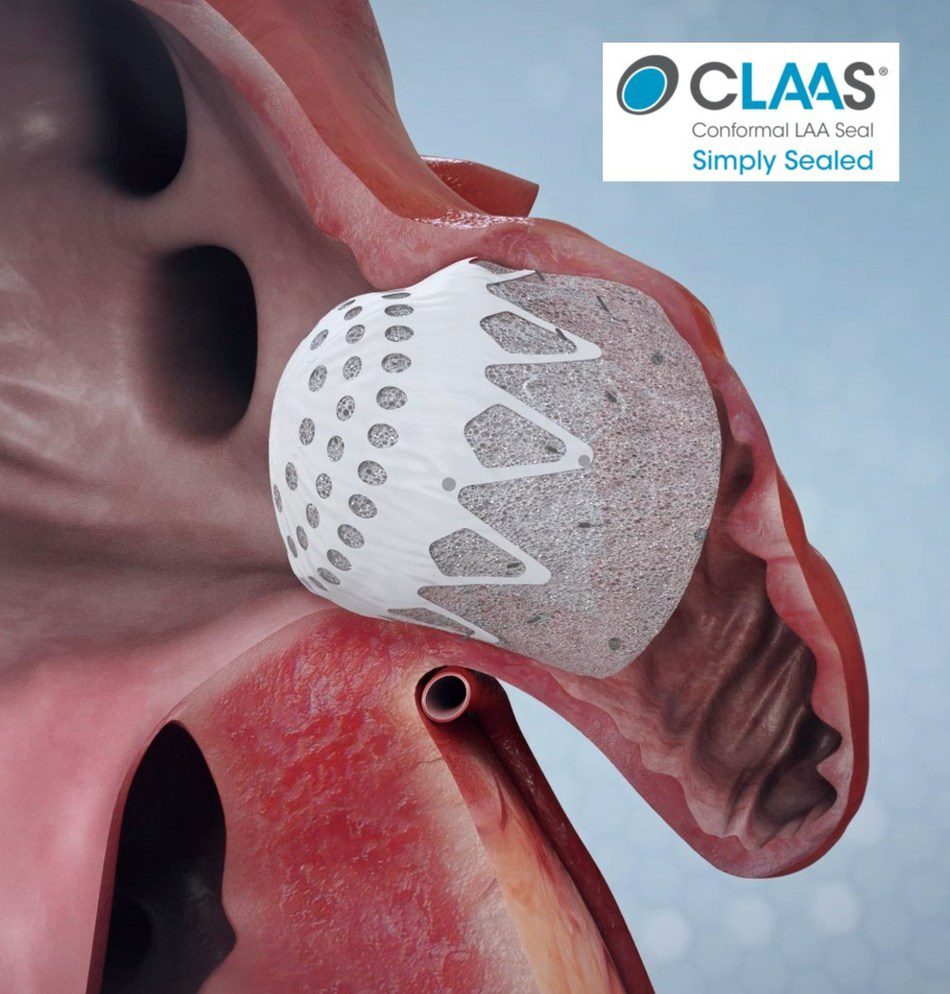
Funds will be used to support the company’s U.S. pivotal trial of its novel CLAAS® Technology, a next-generation solution for sealing the left atrial appendage in patients with atrial fibrillation.
“We are extremely pleased with the strong support of our investment partners, validating the potential of our next-generation technology in the growing LAAC market,” commented Andy Levine, president and CEO of Conformal Medical. “With this significant raise, we look forward to initiating our pivotal trial and taking the next step to establish the CLAAS implant as a cornerstone stroke reduction strategy for patients with AFib.”
“Conformal’s innovative approach has the potential to provide tremendous clinical benefit to a large patient population that suffers from AFib and therefore is at an increased risk of stroke. Current risk-reduction strategies have significant limitations and leave a clear unmet need in this space,” stated Joshua Phillips, managing partner at Catalyst Health Ventures. “The CLAAS technology provides a differentiated solution and we are excited to strengthen our partnership with this proven team and welcome our new funding partners.”
The CLAAS system features a proprietary foam-based architecture designed to accommodate most anatomies with only two sizes and simplified delivery without the need for procedural transesophageal echo (TEE) allowing physicians to perform LAAC without general anesthesia. Conformal’s randomized pivotal trial is expected to enroll over 1,300 patients at multiple sites globally beginning in early 2021, to investigate the safety and effectiveness of the CLAAS technology as compared to Boston Scientific’s Watchman products.
“The left atrial appendage, or LAA, is a pouch off the left atrium where clots associated with stroke form in patients with AFib. First-generation technology has demonstrated effectiveness compared to oral anticoagulation and fueled early market growth, despite procedural limitations,” commented Aaron V. Kaplan, M.D, professor of medicine at Dartmouth and Conformal’s co-founder and chief medical officer. “The CLAAS technology aims to address these limitations and enable physicians to routinely close the LAA without general anesthesia.”
More than six million people in the United States suffer from AFib, placing them at an increased risk of stroke.1 Current standard of care for stroke prevention is chronic oral anticoagulants, which are not well accepted by patients due to concern about associated risk of bleeding. Left Atrial Appendage Closure (LAAC) is emerging as an important alternative to blood thinners for preventing strokes in patients with AFib. Currently, first-generation LAAC devices are an estimated $600M global market and are expected to grow to over $1B in the next five years.
Reference
1. https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_atrial_fibrillation.htm