January 22, 2021
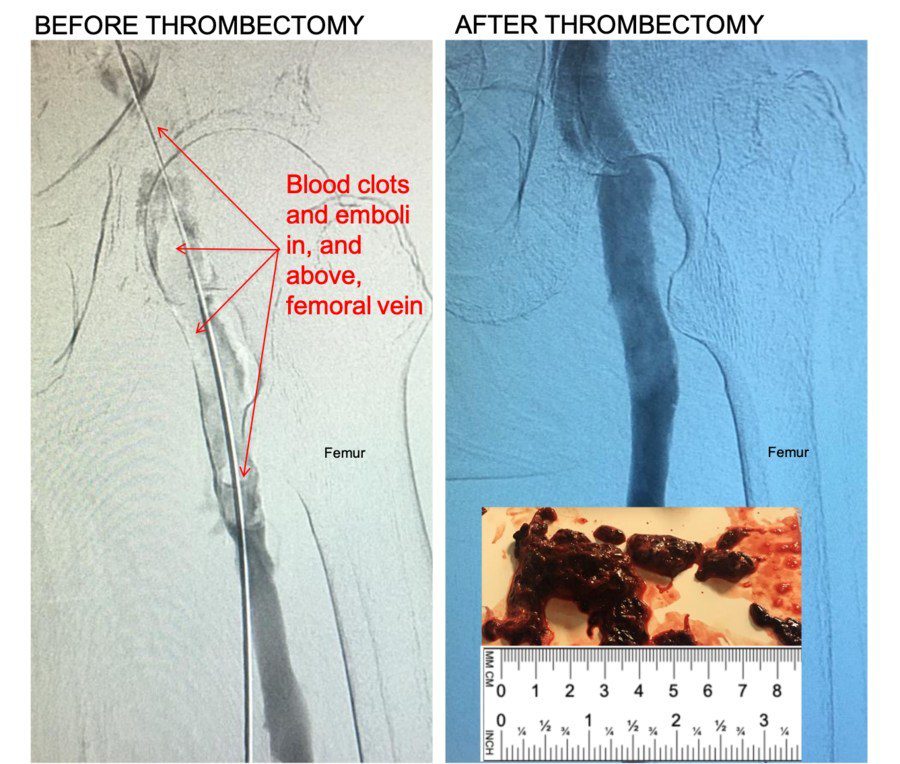
Control 11F Mechanical Thrombectomy System: Kishor Vora, M.D., FACC, FSCAI, CCDS, Medical Director at the Owensboro Heart & Vascular Clinic in Owensboro, Kentucky, USA removed the clots using FDA cleared Control 11F Mechanical Thrombectomy System.
“Control removed large blood clots in our first uses,” said Kishor Vora, M.D., FACC, FSCAI, CCDS, Medical Director at the Owensboro Heart & Vascular Clinic in Owensboro, Kentucky, USA. “Large venous blood clots in the legs is a serious condition. Patients may present with pain, swelling, and other symptoms. Left untreated, deep vein thrombosis (DVT) can lead to life-threatening pulmonary embolism. If clots are not removed from large veins like Common Femoral or Iliac veins in time, it can result in lifelong disabling symptoms like swelling in legs, pain, discoloration and non-healing ulcers.”
Blood-clot and embolic removal, or thrombectomy and embolectomy, is performed throughout the body. Coronary thrombectomy is performed in acute myocardial infarction (AMI), neurovascular thrombectomy in acute ischemic stroke, and peripheral thrombectomy is performed with peripheral arterial disease (PAD), chronic total occlusion (CTO), and deep vein thrombosis (DVT).
“The Control 11 French Mechanical Thrombectomy System can remove large clots,” said Shawn Fojtik, President of Control Medical Technology in Park City, Utah. “Patients, clinicians, and payors need more tools to remove diverse thrombus without excessive expense and complex capital equipment. A disposable $2,000 – $15,000+ device per-patient technology is not the only way to remove blood clots, especially if it sucks too much blood.”
During thrombectomy & embolectomy procedures, clinicians typically track a catheter to the thrombus/emboli and then use a basic syringe or high-performance aspirator/electric pump to pull the clot/emboli out. Basic syringes lack speed, force, volume, and control. Electric pumps improve force but are complex and reported to suck too much blood with 7+ French (~2.25mm+) outer diameter catheters.
“Reducing blood loss is important when removing large clot & emboli,” continued Mr. Fojtik. “Large catheters can remove blood too fast when using electric pumps. Our new FDA cleared system is designed to improved control with a wide range of catheters. We look forward to seeing improved clot targeting, capture, maceration, and removal improve patient care.”
Control Medical’s product platform includes the Control 5 -11 French Mechanical Thrombectomy System with over-the-wire (OTW) catheters indicated for use in the peripheral vasculature and the Control RX-LP Mechanical Thrombectomy System with rapid-exchange (RX) catheters indicated for use in peripheral and coronary vasculature.
The device is distributed here.