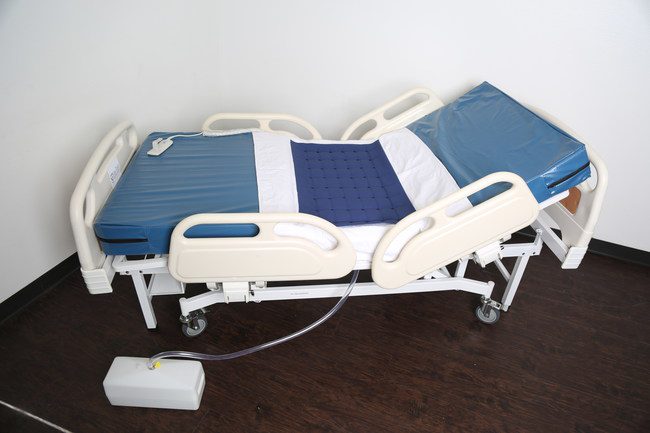
Easy Drain Bed has been launched reports Easy Drain Care Products.
Easy Drain Bed is a fully functional and multi-operational hospital and long-term care bed, the Easy Drain Bed allows patients to rest without having to wear uncomfortable adult-diapers or worry about incontinence.
Each Easy Drain Bed is fitted with a special waterproof mattress that rapidly channels and funnels urine and other fluids through holes into a self-contained hose before landing in a holding tank for easy collection, monitoring and disposal. Easy Drain Care Products LLC is a start-up company that has already become a trusted manufacturer and provider of medical equipment like Easy Drain Mattresses and Cushions in the Dallas and Ft. Worth area.
“We’re so happy to introduce our Easy Drain products into the healthcare market,” said Ezra Nyankira. “It is the solution to an immediate and persistent problem that has just never been adequately addressed by the healthcare industry: urinary and fecal incontinence. The problem is common and widespread and has been traditionally combatted with adult diapers and bedpans, which require frequent changes. Other solutions involve bed, dressing, or gown changes; frequent bathroom trips and re-positioning that increases the risk of fall incidents; and a variety of other solutions which don’t always contribute to the maximum comfort of patients – especially the elderly.
And despite all these approaches, repeated accidents still occur, leading to wet and soiled skin, increased chance for pressure ulcers, and multiple other issues. Our Easy Drain mattress is an incredibly effective solution to this complex problem. Rather than combat the incontinence itself, the mattress simply works around it: funneling urine and fecal matter quickly and safely away from the patient.”
Easy Drain Bed and Mattress: Main Features
- Beds are manual, semi-electric or fully electric; air-inflatable, foam or spring mattress.
- Easily cleaned waterproof mattress with removable center section.
- Strategically located drain tubes in center of mattress, with egg-crate surface section that prevents pooling of fluids.
- Collects urine and fecal matter in easy-to-empty collection tank for fast clean up.
- Saves time, energy and money. Ideal for healthcare facilities and homes.
- Improves overall hygiene of bed-ridden clients, and contributes to quality of life.