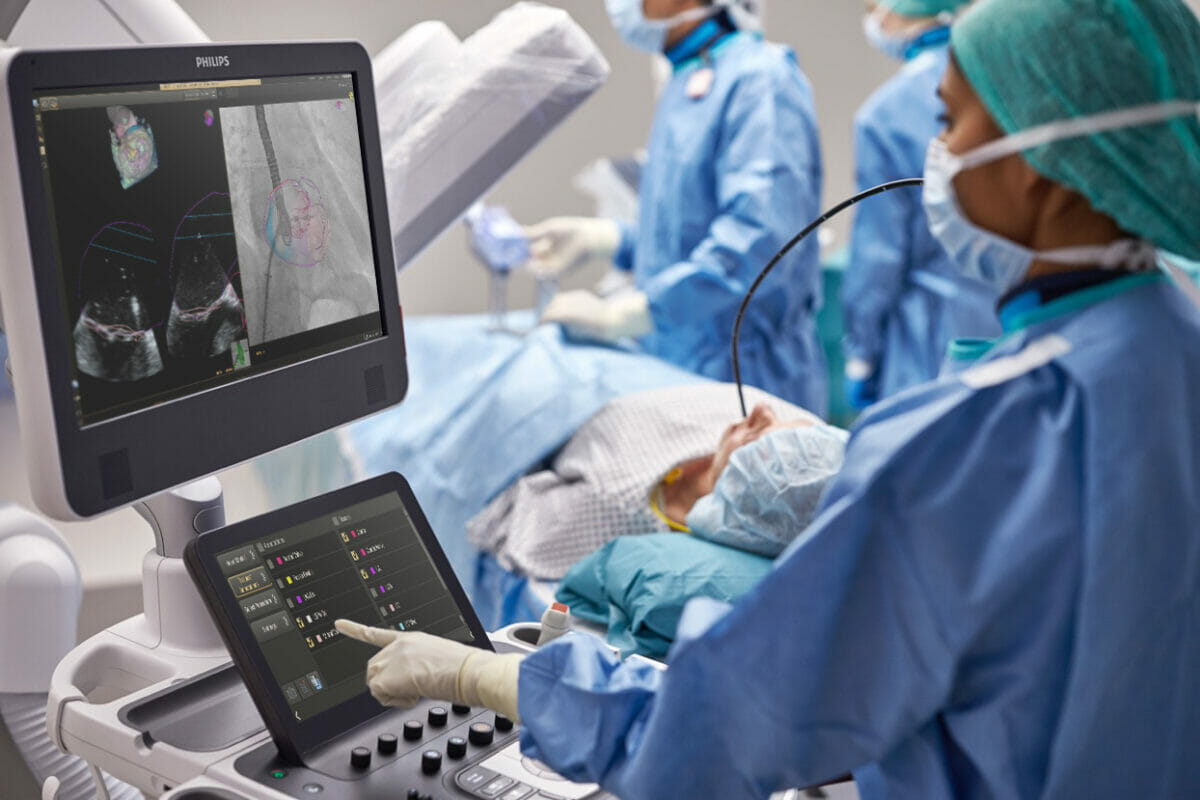
EchoNavigator 4.0: Royal Philips today announced at EuroPCR (17 – 20 May 2022, Paris, France) the international launch of EchoNavigator 4.0 [1], the new release of its advanced image-guided therapy solution for the treatment of structural heart disease.
EchoNavigator 4.0 gives users of Philips’ EPIQ CVXi interventional cardiology ultrasound system greater control of live fusion-imaging on the company’s Image Guided Therapy System – Azurion – platform.
By integrating real-time transesophageal echocardiography (TEE), which places the ultrasound transducer close to the heart, and X-ray fluoroscopy, EchoNavigator 4.0 helps interventional teams to decide, guide, treat, and confirm complex structural heart disease therapy, such as heart valve repair or replacement. The solution also includes extended anatomical intelligence models, transseptal puncture guidance to help access the left atrium and mitral valve from the right atrium, and new 3D live image fusion capabilities, including Philips’ TrueVue photo-realistic rendering and GlassVue volumetric imaging modes. It also features automatic selection of an appropriate set of multiplanar reconstruction planes (sections taken from the 3D echo heart model), with presets for common views of the aortic and mitral valves and left atrial appendage.
“The latest EchoNavigator release gives us unique peri-interventional possibilities by offering a comprehensive set of automated views based on advanced 3D heart models in combination with live fusion imaging,” said Dr. Patric Biaggi, Head of Cardiac Imaging at Heart Clinic Hirslanden in Zurich, Switzerland. “This allows us to treat our patients with greater confidence and precision during every stage of the procedure.”
Largely due to lifestyle choices and the aging population, structural heart disease is now commonplace in older individuals. In the USA, for example, as many as 1 in 10 people over the age of 75-years are affected by a condition known as mitral regurgitation [2], which means the mitral valve in their heart does not close properly, adversely affecting the amount of oxygenated blood that can be pumped round their body. Worldwide, around 156 million people are estimated to suffer from the condition [3]. Fortunately, in many cases treating structural heart disease can now be performed via image-guided, minimally-invasive, catheter-based procedures that impose far less trauma than open-heart surgery.
Improved communication and teamwork
Philips EchoNavigator helps improve communication and teamwork between echocardiographers and interventionists during image-guided therapy by automatically fusing together echocardiography ultrasound and X-ray images, while also enhancing understanding of the relationship between X-ray and ultrasound in an intuitive way that helps interventional teams to complete procedures with greater safety, confidence, and clarity.
“Cardiology teams across the world are facing increasing numbers of complex structural heart disease cases and are seeking new ways to deliver effective high-quality care despite staffing shortages,” said Karim Boussebaa, General Manager of Image Guided Therapy Systems at Philips. “By helping echocardiographers and interventionists to work together in even more highly coordinated ways, this new release of EchoNavigator is an important step forward in boosting patient throughput, making more efficient use of time and resources, and achieving positive cardiovascular care outcomes.”
Greater control for echocardiographers
Extended automatic modeling and transseptal puncture guidance
Enhanced fusion imaging with Cardiac TrueVue and TrueVue Glass rendering
EchoNavigator 4.0’s fusion imaging capabilities have been further enhanced by the ability to fuse live X-ray fluoroscopy images with live TrueVue and TrueVue Glass rendered echocardiography images to more easily visualize positioning and device-tissue interactions. Philips TrueVue 3D echo rendering improves visualization of anatomical structures and devices, while TrueVue Glass with Color allows interventionists to view the location of regurgitant blood flow across a heart valve.
With its long history of leadership in cardiology innovation, informed by continuous collaboration with leading clinical partners, Philips is uniquely positioned to deliver integrated solutions that span the care pathway to help solve cardiology’s daily challenges and provide better heart care with greater efficiency.
[1] Philips EchoNavigator 4.0 is not yet available in all markets, e.g not available in USA or China.
[2] Lloyd-Jones D, Adams RJ, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association https://pubmed.ncbi.nlm.nih.gov/20019324/.
[3] Dziadzko V, Clavel MA, Dziadzko M, et al. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet. 2018;391(10124):960-969. doi:10.1016/S0140-6736(18)30473-2 https://pubmed.ncbi.nlm.nih.gov/29536860/.