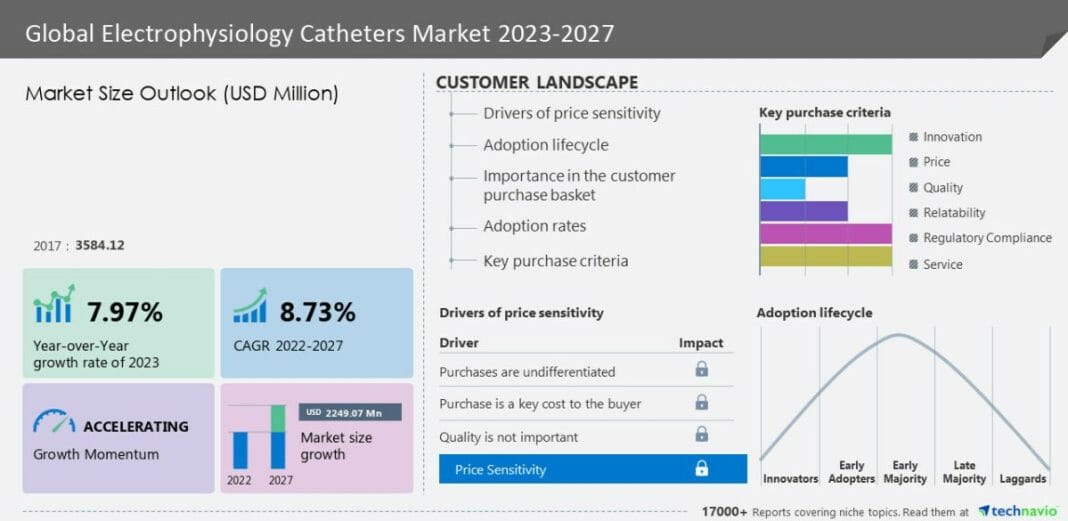
The Electrophysiology Catheters Market size is expected to grow by USD 2.24 billion, accelerating at a CAGR of 8.73% during the forecast period, according to Technavio Research. Companies are implementing various strategies like forming partnerships, mergers, expanding into new areas, and launching new products to improve their market position. The report also offers detailed analyses of the market’s competitive landscape, featuring information on leading companies including Abbott Laboratories, Acutus Medical Inc., AngioDynamics Inc., APT Medical, AtriCure Inc., BIOTRONIK SE and Co. KG, Boston Scientific Corp., CardioFocus Inc., CathRx Pty Ltd., Japan Lifeline Co. Ltd., Johnson and Johnson, Koninklijke Philips N.V., Medtronic Plc, Merit Medical Systems Inc., MicroPort Scientific Corp., Millar Inc., Nihon Kohden Corp., OSYPKA AG, Siemens Healthineers AG, Stereotaxis Inc. and Stryker Corp. This report offers an up-to-date analysis of the current market scenario, the latest trends and drivers, and the overall market environment.
Electrophysiology Catheters Market: Company Profile
Abbott Laboratories: The company offers electrophysiology catheters such as Advisor HD grid sensor-enabled mapping catheter.
To gain access to more company profiles available with Technavio, buy the report
Electrophysiology Catheters Market: Segmentation Analysis
The market is segmented by End-user (Hospitals and cardiac centers and ASCs), Product (Ablation catheters and Diagnostic catheters), and Geography (North America, Europe, Asia, and Rest of World (ROW)).
Learn about the contribution of each segment summarized in concise infographics and thorough descriptions. Download the free sample report here
Electrophysiology Catheters Market: Geographical Analysis
It is projected that North America will play a substantial role, contributing approximately 38% to the global market’s growth during the forecast period. In developed nations within the region, notably the United States and Canada, the presence of well-established healthcare facilities is driving increased demand for balloon catheters and cardiac ablation catheters.
Electrophysiology Catheters Market: Driver, Trend & Challenge
Driver – The growth of the electrophysiology catheter market is driven by the increasing incidence of cardiac diseases and the expansion of insurance providers. Heart disease prevalence is rising globally, including valvular heart disease, especially due to increased life expectancy. High blood pressure is a leading cause of conditions like atrial fibrillation, which can lead to strokes or heart attacks. Additionally, healthcare options like the European Health Insurance Card (EHIC) in the EU and medical screenings provided by the UK’s National Health Service (NHS) further contribute to market growth.
Trends – The use of remote navigation in cardiac mapping and imaging is the key trend in the market.
Challenges – A stringent regulatory framework is a challenge that affects the growth of the market.
Identify more information on key trends, drivers, and challenges of the market. Download to gain access to this information
What are the key data covered in this electrophysiology catheters market report?
- CAGR of the market during the forecast period
- Detailed information on factors that will drive the growth of the electrophysiology catheters market between 2022 and 2027.
- Precise estimation of the electrophysiology catheters market size and its contribution to the market in focus on the parent market
- Accurate predictions about upcoming trends and changes in consumer behavior
- Growth of the market across North America, Europe, Asia, and ROW
- A thorough analysis of the market’s competitive landscape and detailed information about vendors
- Comprehensive analysis of factors that will challenge the growth of electrophysiology catheters market vendors.