Engenious Design has partnered with Manhattan (KS) startup Precision Microwave to develop a technologically-advanced system to treat previously-inoperable cancer tumors with minimally-invasive surgery.
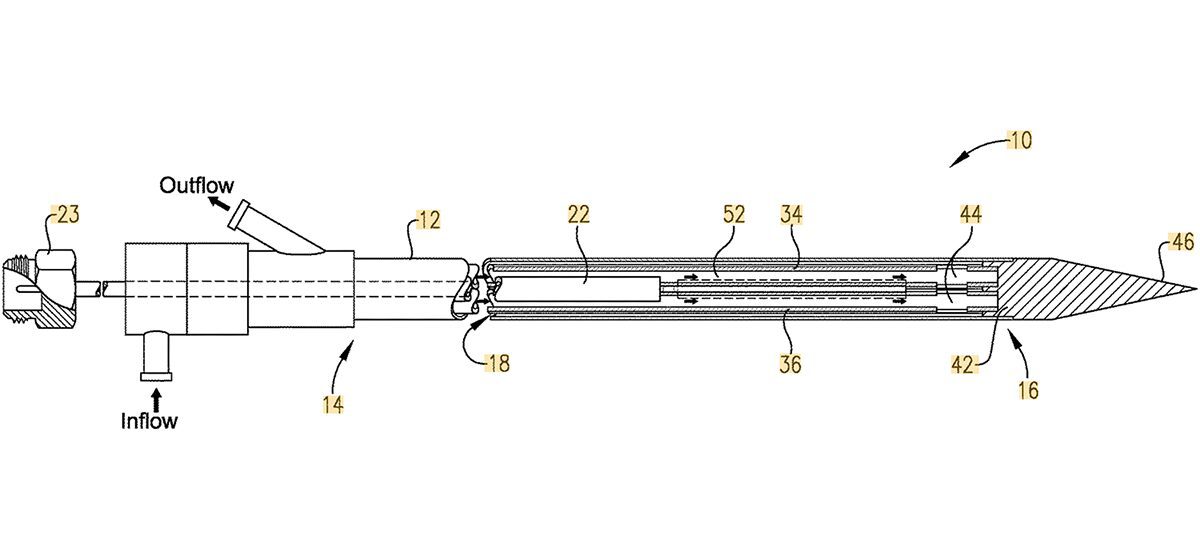
The system is a new Microwave (MW) Generator to accompany Precision Microwave’s next-generation Directional Microwave Ablation (DMWA) applicator.
Precision Microwave and Engenious Design are combining Precision’s market-disruptive technology with Engenious Design’s experience in medical device design. Precision Microwave’s DWMA system is a new tool for minimally-invasive thermal ablation treatment of cancers, a novel treatment tool with ability to reduce the risk of unintended injury to vital structures near tumors. The new technology complements existing options for thermal ablation and greatly expands the tools available to surgeons that address challenging cases and broaden the range of cases that could be safely and effectively treated.
Austin Pfannenstiel, Precision Microwave’s founder and CEO, explains, “Engenious Design has been the perfect partner for a small, start-up medical device company like us. I have been consistently impressed by their team’s tremendous depth of knowledge and experience spanning technical & industrial design, clinical insight, and regulatory considerations, which has enabled us to stay focused on leveraging our own strengths while making significant progress towards commercialization. Importantly, Engenious Design has always been very considerate of the resource constraints faced by a small start-up and has been invaluable in helping us identify and accomplish milestones that add maximum value.”
Precision Microwave’s system was recently granted a key patent protecting the unique, directional ablation technology. Precision Microwave’s regulatory strategy also employs a faster and lower-risk path to FDA clearance by leveraging predicate devices. Precision Microwave has already received $1.2 MM in grant funding from the National Science Foundation (NSF), and are working to close additional funds to pursue FDA clearance and commercialization.
Chris Justice, Principal at Engenious Design, adds, “Most of us have been affected by cancer through family, friends or our own experience, and we are delighted to help Precision Microwave create better tools to fight cancer. Precision Microwave’s technology is truly innovative and works to provide more precise control to surgeons; control that allows targeting of cancers and preservation of adjacent vital structures. The Precision Microwave team has developed technology that is a game-changer in cancer therapy and we are excited to partner and bring this technology to market quickly.”
About Engenious Design: Kansas City metro based, Engenious Design is a creative product design firm specializing in electronic medical device design. Since its founding in 2013 by spouses Chris and Holly Justice, the Engenious Design team has grown from 2 to 40+ team members. Team expertise includes Electrical, Embedded Software, Mechanical and Test Engineers, Industrial, Interaction, and Graphic Designers, as well as others. Engenious Design capabilities include an on-site electronics lab, quick-turn model shop, and short-run production capabilities with a full medical device quality management system.