"Accuracy and precision across the entire image generation chain, data processing speed and native AI-based application management lie at the heart of MyLab™X90," ultrasound system said Guillaume Gauthier, Ultrasound Global Product Marketing Manager.
Esaote Presents MyLab™X90 – New Premium Ultrasound System
Linkedin
Twitter
WhatsApp
Facebook
Copy Link
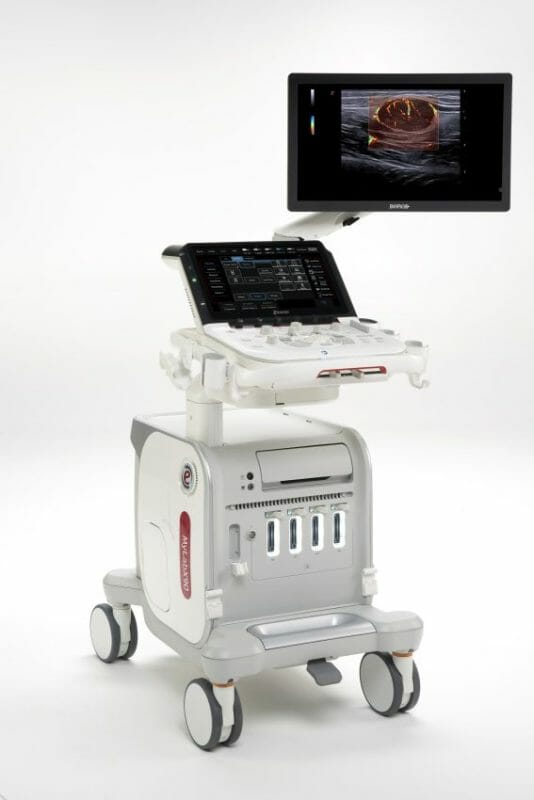
Today Esaote, a leading Italian company in the biomedical sector – in ultrasound, dedicated MRI and medical IT– presents the world premiere of MyLab™X90, its new premium ultrasound system.
“Accuracy and precision across the entire image generation chain, data processing speed and native AI-based application management lie at the heart of MyLab™X90,” said Guillaume Gauthier, Ultrasound Global Product Marketing Manager. “As well as providing high-quality images and advanced clinical solutions, our aim was to revolutionize daily workflows by increasing the speed and accuracy of analysis, together with improving the operator experience and the clinical outcome for patients.”
MyLab™X90 offers premium components, where the starting points are the renewal of the XCrystal probe family and the exclusive dual-layer eLed monitor by Barco. These ensure outstanding on-screen image display at a very high contrast resolution, far superior to other product lines.
MyLab™X90 also offers advanced ergonomics in clinical solutions focused on the operator, which extend data analysis capacity and ensure a unique customer experience based on diagnostic confidence, as well as on automation to improve the usability and availability of advanced technologies in everyday clinical practice.
“We are highly satisfied with this outcome, the result of substantial investment and intense work on R&D. The new MyLab™X90, with its all-Italian design, represents a further evolution in the range of technologies we provide to assist with people’s care and well-being,” noted Franco Fontana, CEO of Esaote. “It represents the upshot of strategic investment and of our international, multidisciplinary team, who can tackle the challenges of innovation and anticipate requirements in the most advanced and interconnected healthcare systems. We believe that these aspects, combined with the latest technologies, can contribute significantly to improving the level of patient care, and are delighted to play an active role.”
For its world premiere Esaote selected Manama, an international context and a vibrant multicultural hub. The event, that will be attended by the Bahraini authorities and by international clinical experts will be livestreamed on Esaote LinkedIn page (https://www.linkedin.com/events/mylab-x90livestreaming-a-i-augm7021420599726026753/) and will then be available on demand at mylabx90.com .
The new MyLab™X90 will be showcased at Arab Health 2023 (Dubai, January 30 – February 2), and at the European Congress of Radiology (ECR) 2023, in Vienna from March 1 to 5.
510(k) pending at FDA, not available for sale in USA.