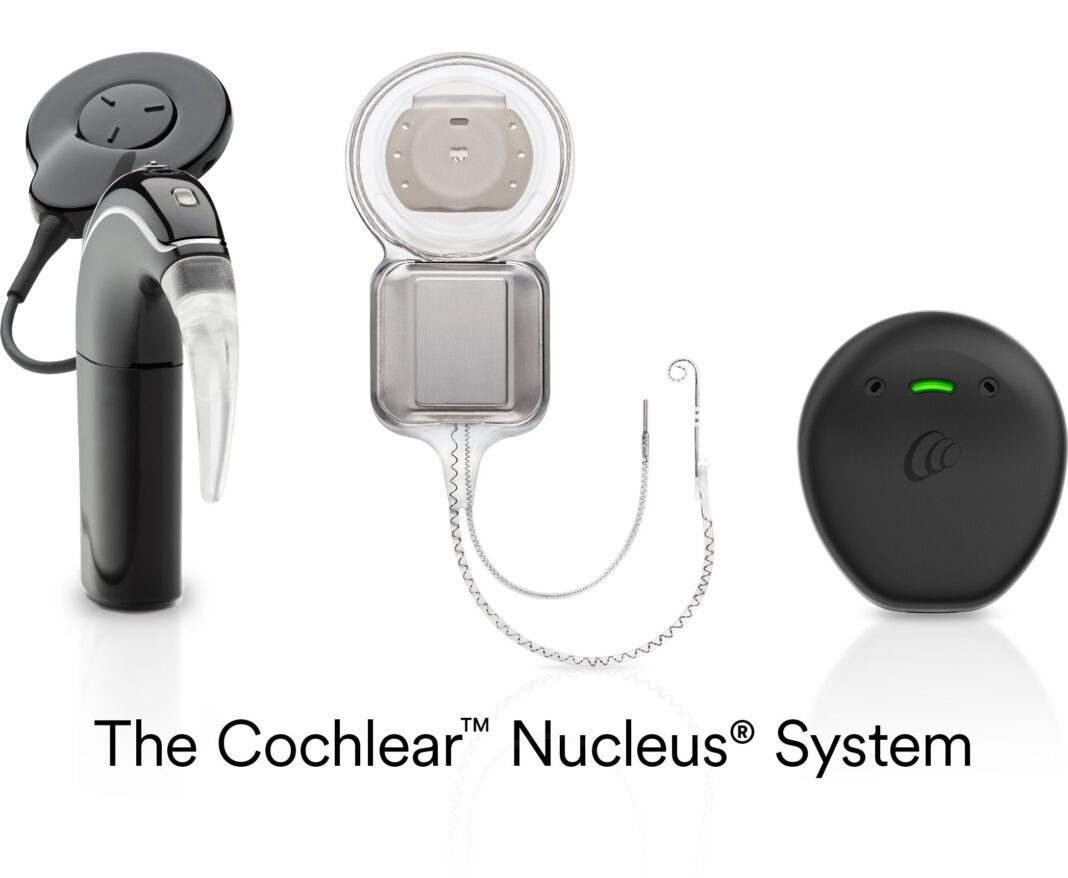
Cochlear Limited (ASX: COH), the global leader in implantable hearing solutions, obtained U.S. Food and Drug Administration (FDA) approval of Cochlear™ Nucleus® Implants for the treatment of unilateral hearing loss (UHL)/single-sided deafness (SSD).
Cochlear implants are already FDA approved for those with moderate to profound bilateral sensorineural hearing loss. With this approval, for the first time Cochlear can expand implantable treatment options for those with UHL/SSD to include cochlear implants.
UHL is classified as hearing loss in one ear and near to near-normal hearing in the opposite ear, and SSD is specific to individuals with severe to profound hearing loss in one ear and normal or near-normal hearing in the other ear. Every year, about 60,000 people in the United States acquire SSD.1
“It is not often that approvals to expand indications and increase awareness about effective treatments for hearing loss come along. Now with this approval, Cochlear is proud to offer the most hearing implant options available to those with unilateral hearing loss/single-sided deafness through our cochlear implant and bone conduction solutions,” said Christine Menapace, MA, Vice President, Clinical Affairs, Cochlear Americas. “It is important that those with this type of hearing loss recognize the impact to their lives and understand there are several options available to them, and we encourage them to talk to their hearing health professional today to find out what would work best for them.”
UHL/SSD can impact an individual’s quality of life, including mental fatigue because of increased listening effort, as well as impacts to understanding speech in noise and localizing where sounds are coming from.2-6 Hearing with both ears is important. Hearing with two ears helps one to identify sounds both near and far, as well as those that occur 360 degrees around the head.7
“Unilateral sensorineural hearing loss has continued to gain greater acceptance in the field as an indication for cochlear implantation,” said Dr. Kevin Brown, Vice Chair, Chief of Neurotology and Executive Director of the Children’s Cochlear Implant Center at the University of North Carolina. “With this approval, it is important my industry colleagues and I ensure appropriate clinical guidance on how to effectively introduce this cochlear implant indication, outline a model for selecting patients that will benefit, and ensure success after implantation.”
Cochlear will now support those with UHL/SSD by offering its cochlear implant, the Cochlear Nucleus System, and bone conduction solutions, the Cochlear Osia® System and the Cochlear Baha® System. Cochlear’s implantable hearing solutions restore access to sound to improve quality of life and meet the lifestyle needs and preferences of each individual recipient.