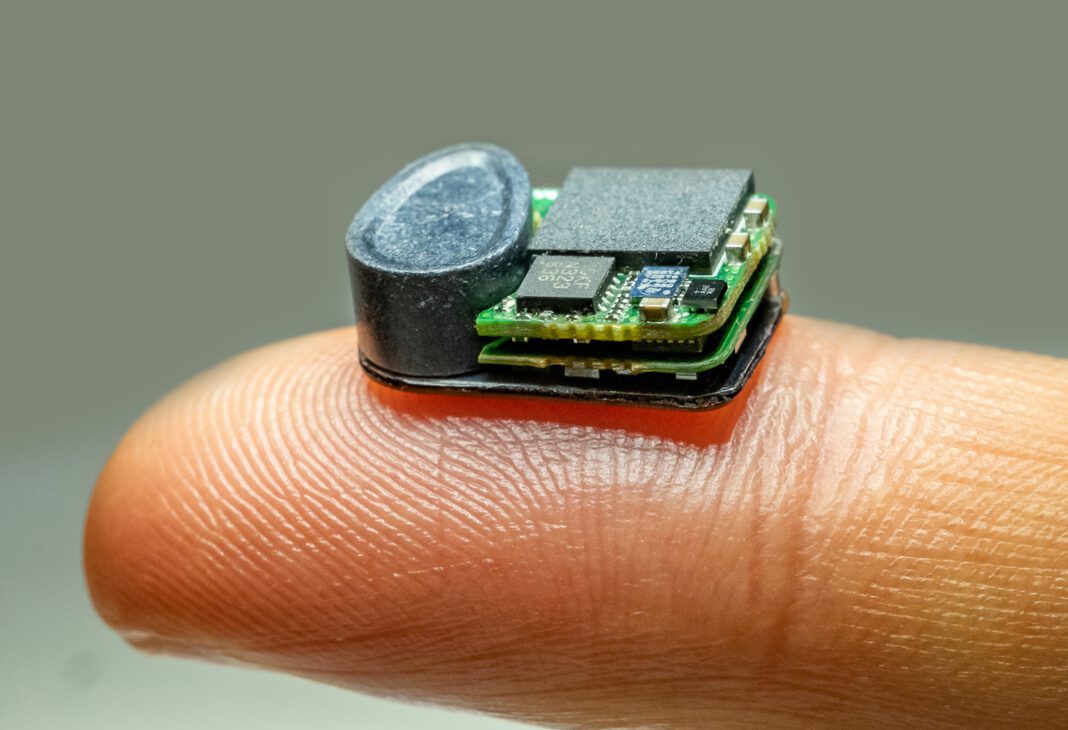
Engineers and researchers in the Institute of Bioelectronic Medicine at The Feinstein Institutes for Medical Research have developed a novel fully-implantable wireless bidirectional vagus nerve stimulation (VNS) and sensing device for mice. Details about the device and its capabilities, published in the journal Biosensors and Bioelectronics, outline the potential to transform how bioelectronic medicine research is conducted in labs worldwide.
Bioelectronic medicine is a new approach to treating and diagnosing disease and injury that has emerged from the Feinstein Institutes’ labs. The emerging field of science represents a convergence of neuroimmunology and technology, which uses electricity to stimulate the nervous system, particularly the vagus nerve. Through this electrical stimulation – known as neuromodulation – scientists study how the brain and organs communicate to control things like inflammation, heart rate, and oxygen. Early research performed in mice may lead to the discovery of alternative therapies for various chronic diseases, including Crohn’s, rheumatoid arthritis and heart failure. Unlike pharmaceuticals, using devices in mice presents a number of surgical and technological challenges. It has seen limited research progress, resulting in many groundbreaking discoveries of the past few decades failing to reach clinical application.
“Neuromodulation and bioelectronic medicine hold the potential to treat a variety of diseases without the use of traditional pharmaceuticals and their potential side effects, however, scaling devices to the size needed for studies in mice has been very challenging,” said Timir Datta, PhD, assistant professor in the Institute of Bioelectronic Medicine at the Feinstein Institutes and senior author on the paper. “This new device will finally enable the fundamental preclinical studies needed to inform future clinical trials that could lead to potentially revolutionary bioelectronic therapies for patients in need.”
Dr. Datta and his team designed and built the new device using only off-the-shelf, commercially available components and 3D-printed parts to ensure other researchers can also build and use this system. The paper describes how the device was used to apply VNS, and sense physiological and neural signals.
Bioelectronic medicine research has developed advanced techniques for creating mouse models of human diseases, but engineering limitations impact the ability to study the long-term effects of device-based therapies. The new device is compatible with different types of electrodes and sensors and can be used for applications other than VNS, such as deep brain stimulation or pain relief. Importantly, the device is also wirelessly rechargeable while the mouse remains completely free to move and interact with its environment, which will allow researchers to design long-term studies to look at disease treatment progression.
The team’s research builds off previous studies unveiled by the Feinstein Institutes – the global scientific home of bioelectronic medicine. In 2020, Feinstein researchers showed the effective use of a long-term vagus nerve implant in mice as well as a paper that describes the surgical technique to implant a mico-cuff electrode onto a mouse cervical vagus nerve and evaluating its effectiveness in suppressing inflammation.
“Basic science in mice is foundational to developing new devices and effective treatments for patients,” said Kevin J. Tracey, MD, president and CEO of the Feinstein Institutes. “By offering new methods for other labs to use in their neuromodulation work, Dr. Datta and his team are leading the way to new discoveries in neuroimmunology and bioelectronic medicine research.”