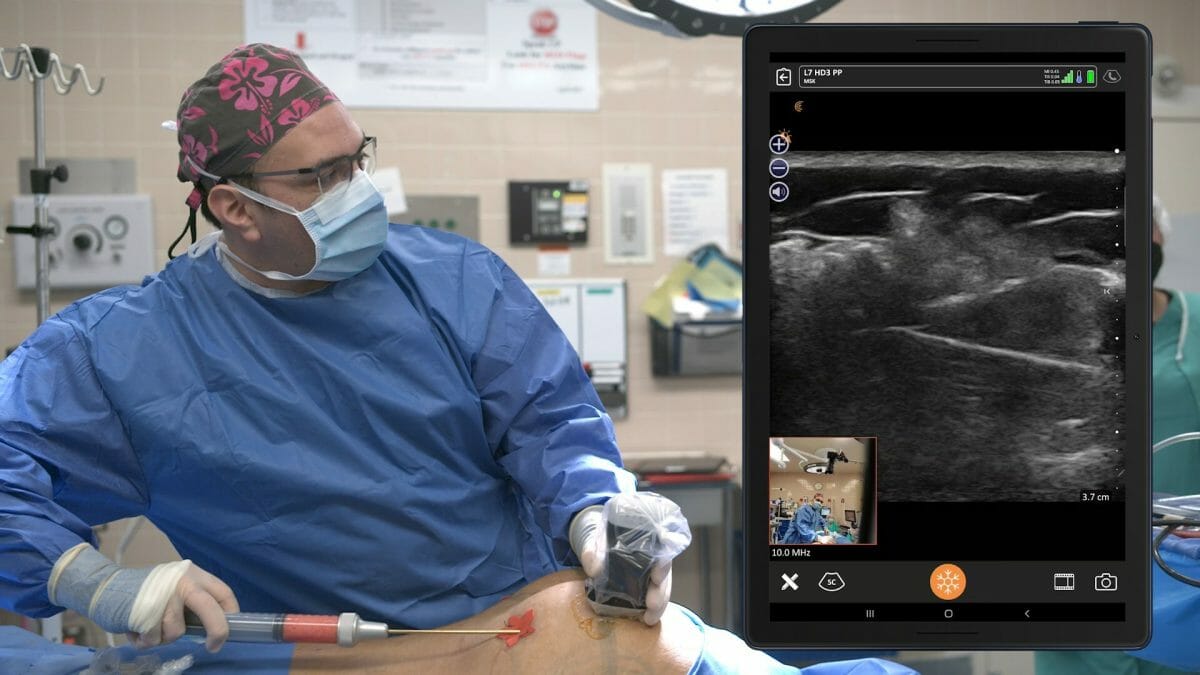
As of July 1, 2023, Florida physicians who perform gluteal fat grafting must use ultrasound guidance to clearly see that fat is injected only in the subcutaneous space and never into the muscle. When fat grafts have inadvertently been injected into the gluteal muscle, the fat has traveled to the heart, lungs, and brain with fatal results. Dedicated to improving patient safety, Clarius is pleased to announce that today the Clarius L7 HD3 is the leading wireless ultrasound scanner among Florida's plastic surgeons.
Florida Passes Plastic Surgery Law Mandating Use of Ultrasound Guidance to Help Ensure Safety of Gluteal Fat Grafts
Linkedin
Twitter
WhatsApp
Facebook
Copy Link
Florida’s House and Senate recently passed the state’s first plastic surgery bill, HB 1471, which mandates the use of ultrasound guidance during gluteal fat grafting procedures to protect patient safety. This bill was sent to the governor’s office who signed it into law. As of July 1, 2023, Florida physicians who perform gluteal fat grafting must use ultrasound guidance to clearly see that fat is injected only in the subcutaneous space and never into the muscle. When fat grafts have inadvertently been injected into the gluteal muscle, the fat has traveled to the heart, lungs, and brain with fatal results. Dedicated to improving patient safety, Clarius is pleased to announce that today the Clarius L7 HD3 is the leading wireless ultrasound scanner among Florida’s plastic surgeons.
“We’re elated that ultrasound is now the law of the land in Florida for BBLs. This is the first time a plastic surgery law has passed,” says renowned plastic surgeon Dr. Pat Pazmiño MD, FACS. “The Florida Society of Plastic Surgeons has worked for decades as advocates for patient safety. We were honored to sponsor this bill and lobby government officials to protect patients. We are grateful that Governor DeSantis signed our bill into law and helped make legislative history! And we’re also thankful to the team at Clarius for their incredible help and support.”
Gluteal fat grafts, or Brazilian Butt Lifts (BBLs) as they are commonly known, are considered to have one of the highest mortality rates of any cosmetic surgery, according to the American Society of Plastic Surgeons. To ensure safer procedures, the new Florida law also mandates that surgeons cannot meet the patient for the first time on the day of the surgery and that a surgeon cannot delegate the surgical procedure to a nurse or an assistant.
Dr. Pazmiño has used ultrasound at his Miami practice for more than a decade and created the ultraBBL™ procedure, which combines innovative aesthetic surgery techniques with ultrasound guidance to ensure the safest outcomes. He currently uses the Clarius L7 HD3 wireless ultrasound, which is considered the leading choice for plastic surgeons due to its affordability, ease of use, and high-resolution imaging powered by artificial intelligence.
Amanda Leyva, a patient who underwent BBL surgery a year ago, says she thought long and hard before undergoing the procedure. Her research to find the best plastic surgeon in Miami led her to Dr. Pazmiño. “I was really excited to meet with him in person for a consult as most plastic surgeons in the area just want you to send in photos and then you go in for surgery before you’ve met them,” she said in a recent video interview. “We talked about the procedure and about how he does it a little bit differently with the ultrasound technology that it makes it so much safer. Knowing that Dr. Pazmiño uses ultrasound gave me the confidence that I would be ok. It gave me exactly what I was looking for. There were no complications, everything went really well.”
The new Florida State law follows the adoption of the 90-day Emergency Rule (64B8ER22-3) filed by the Board of Medicine and authorized by the Florida Department of State on June 14th, 2022. It also mandated the use of “ultrasound guidance when placing and navigating the cannula and injecting fat into the subcutaneous space,” and furthermore, required the surgeon to “maintain the ultrasound video recording in the patient’s medical record including the time and the date stamp.”
“Within a month of the Emergency Rule, roughly half of the plastic surgeons in the state acquired a Clarius wireless scanner, rather than canceling surgeries,” reports Clarius President and CEO, Ohad Arazi. “The Clarius L7 HD3 delivers high-resolution imaging to improve procedural safety in an affordable ultrasound solution that features advanced plastic surgery software, a dedicated BBL Preset, and Procedure Recording Mode to help surgeons stay ahead of evolving regulations. At Clarius, we’re grateful for our close collaboration with trailblazing physicians like Dr. Pat Pazmiño who help pave the way for better patient safety.”
Plastic surgeons are invited to visit the Clarius Classroom and webinar center for FREE video resources to learn industry leading ultrasound best practices. They feature renowned plastic surgeons like Dr. Pat Pazmiño, Dr. Marc Salzman, Dr. Steven Weiner, Dr. Alexis Delobaux, and many others who share best practices for improving patient safety across a variety of aesthetic procedures, from BBLs, to breast surveillance, to facial fillers.