FX Solutions received 510k clearance for its lateralized and augmented glenoid baseplates. With these additions, FX brings ten additional glenoid baseplate options to the market.
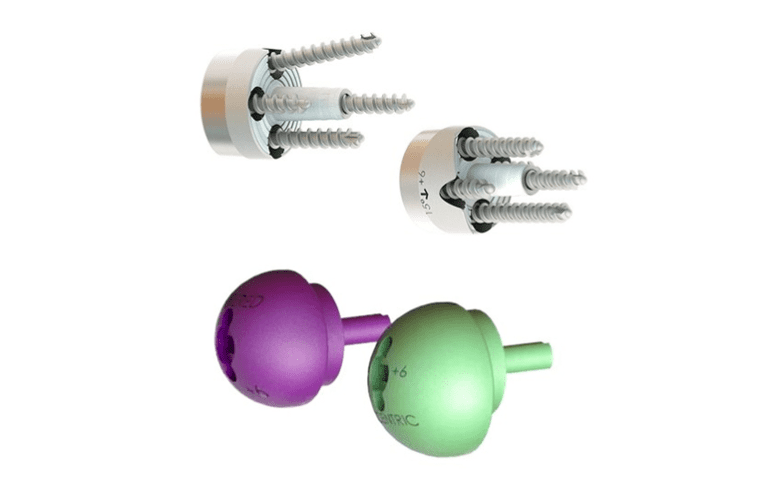
The glenoid baseplates are available in 24mm options with additional lateralization, augments, and lateralized augments. The lateralized glenoid baseplates are available with a central screw or central post option. The augmented glenoid baseplates are available with the central screw option only.
The Humelock glenoid baseplate is a 24mm round baseplate available in four options: baseplate with a central cannulated post, baseplate with a central screw (with seven 4.5mm central screw length options from 8-20mm in 2mm increments), lateralized baseplate with +3mm or +6mm added, and augmented baseplate with 7.5° (3mm thick) or 15° (6.5mm thick) half-wedge added, +0, +3mm, or +6mm lateralized. Lateralization and augmentation are added under the flat surface of the baseplate and above the post.
“As we continue to grow the FX portfolio, we evolve ever closer to providing a tailor-made approach for surgeons using our portfolio. They may be able to adapt the system to the patient rather than the patient to our system. This clearance of the lateralized and augmented glenoid baseplates is truly significant for our portfolio and the treatment of patients’ shoulders,” said Baptiste Martin, CEO of FX Shoulder USA.
With the launch of the lateralized glenoid baseplates FX will include upgrades of their current instrumentation to include a first-to-market and completely unique patent-pending trialing system for the reverse glenoid construct. This features a monobloc trial consisting of the glenosphere and lateralized glenoid baseplate together to better replicate the feel of the definitive implants.
FX continues to innovate based upon surgeon feedback and market trends with a focus exclusively on shoulder arthroplasty with the goal of becoming a global market leader in this segment.