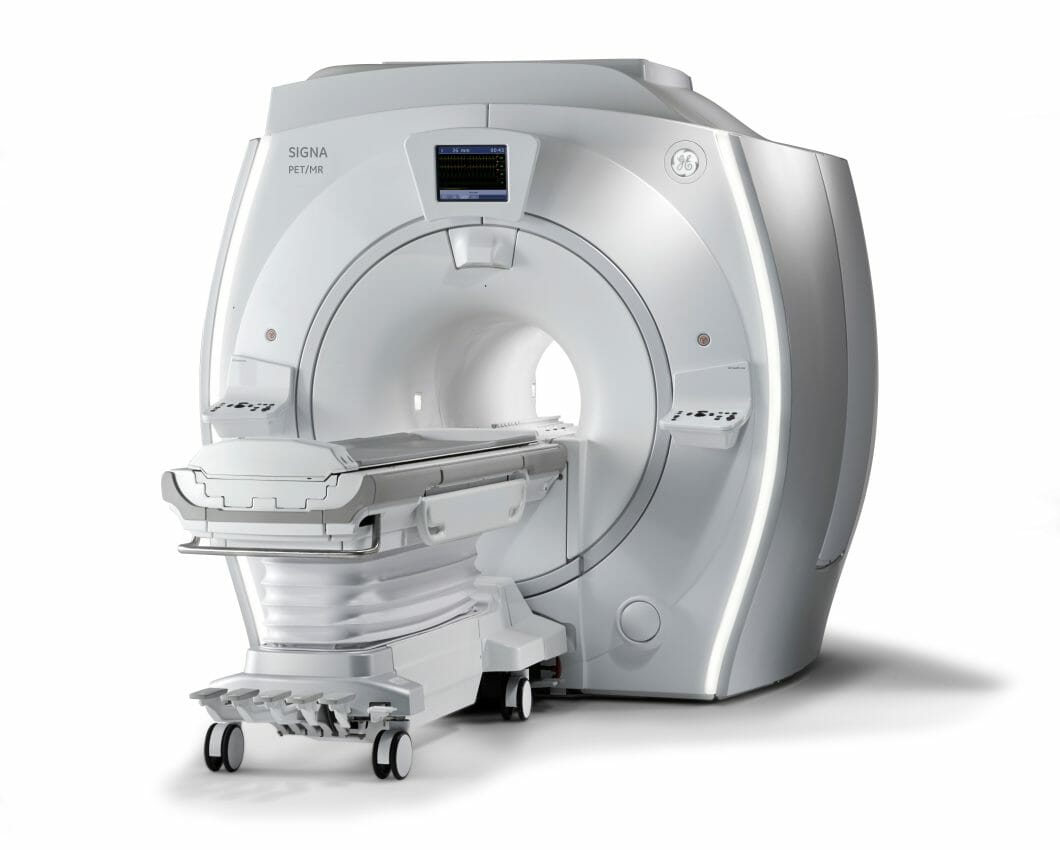
GE HealthCare is set to unveil SIGNA PET/MR AIRi, at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2023 annual meeting. The company will showcase the integration of its advanced AIR technologies with the SIGNA PET/MR AIR system, to enhance diagnostic precision, simplify treatment evaluation, while elevating patient comfort.
Recent FDA approvals of new PET radiotracers and therapeutic approaches for high-prevalence diseases, such as prostate cancer and Alzheimer’s Disease, emphasize the need for reliable and comprehensive imaging solutions throughout the patient care journey. The unique AIR technologies from GE HealthCare incorporated into SIGNA PET/MR AIR address the evolving demands of these patient populations. These technologies include AIR Coils, which enhance and improve patient comfort, AIR Recon DL, which improves MR image quality and enables scan time reduction, and MotionFree Brainii, which mitigates motion-related PET image degradation.
Breakthroughs for Prostate Cancer Diagnosis and Treatment
Prostate cancer is the second most prevalent cancer in the world. Recently, prostate cancer care has undergone a transformation with the introduction of prostate-specific membrane antigen (PSMA)-targeted radiotracers for imaging diagnostics and theranostics, which combines diagnosis and therapy. The integration of PSMA-targeted PET radiotracers with GE HealthCare’s high sensitivity PET and the anatomical precision of MRI in SIGNA PET/MR AIR holds the potential for precise diagnosis and staging/re-staging of prostate cancer. Additionally, it can aid in the selection of patients for subsequent PSMA-targeted radioligand therapyiii.
SIGNA PET/MR AIR delivers deep learning based MR image reconstruction with AIR Recon DL, helping to ensure both improved image quality and up to 50% in reduction of scan timeiv. GE HealthCare’s AIR Coil technology effortlessly conforms to the shape of the human body, enhancing the quality of MR imaging while enhancing patient comfort. This technology offers a thinner, lighter solution, allowing for more accurate PET quantitation in contrast to conventional rigid RF coils.
Overcoming Barriers and Shaping the Future of Alzheimer’s
With a staggering population of approximately 6.7 million Americans and 55 million worldwide affected by Alzheimer’s Disease, GE HealthCare is committed to delivering products and solutions for accurate diagnosis, effective therapy planning, and continuous monitoring of the diseasev.
The recent approval of drugs with disease modifying potential require additional imaging of patients with both MR and PET. SIGNA PET/MR AIR offers the industry’s most sensitive Time-of-Flight (ToF) PET detector in a PET/MR, enabling clinicians to achieve the earliest possible diagnosis, gain insights into disease progression, and detect adverse effects in a single scan.
Addressing the challenge of patient movement during imaging exams, MotionFree Brain PET is a breakthrough solution on SIGNA PET/MR AIR that manages motion without external tracking devices. This helps to ensure consistent image quality even in challenging patients, potentially enabling faster and more accurate diagnosis, and minimizes the potential need for repeat scans.
“The SIGNA PET/MR AIR system continues our legacy of delivering innovative technologies that address a comprehensive range of clinical diseases,” said Jie Xue, President and CEO of MR at GE HealthCare. “Our latest PET/MR technology will directly impact the most challenging diseases, such as prostate cancer and Alzheimer’s Disease. These diseases require precise and comprehensive imaging for accurate diagnosis, treatment planning, and therapy monitoring. We are excited to see SIGNA PET/MR AIR addressing these challenges and fulfilling our vision of providing access to advanced, personalized care.”
The introduction of the SIGNA PET/MR AIR system demonstrates GE HealthCare’s commitment to empowering healthcare professionals to deliver exceptional care and improve patient outcomes in the ever-evolving landscape of medical imaging.
_______________________
i Not CE Marked. Not available for sale in all regions. SIGNA PET/MR AIR is a premium configuration of SIGNA PET/MR
ii Not CE Marked. Not available for sale in all regions.
iii “Cancer.” World Health Organization. Feb 3, 2022. Accessed Jun 1, 2022. https://www.who.int/news-room/fact-sheets/detail/cancer
iv GE HealthCare Data on File
v https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf