September 2, 2020
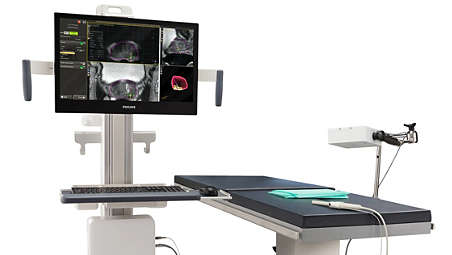
Good Samaritan Medical Center Delray, Florida, is now offering MRI precision targeting for prostate biopsy guidance, a new tool for physicians to use to help men in the fight against prostate cancer. The UroNav Fusion Biopsy System by Phillips is a fully MRI-compatible interventional device for Trans-Rectal Interventional MRI of the prostate gland.
Good Samaritan Medical Center notes it adjusts in six directions for precision targeting and works with DynaCAD prostate to provide less invasive, targeted guidance. The multiple directional adjustments help clinicians access all areas of the prostate gland. Once adjusted, the device can be locked into position, providing added confidence to biopsy procedures.
“This equipment is ushering in a new era in how we treat our patients, and it is designed to give them highly accurate readings for prostate biopsies,” said Dr. Antonio Beltran, urologist and member of the medical staff at Good Samaritan Center. “This new offering provides our patients with one of the latest advanced medical technologies in urology.”
The UroNav device is comprised of three simple components:
- A baseplate that adapts to the MRI gantry.
- A clampstand which connects to the baseplate and offers multiple precise adjustments for location targeting.
- A sterile, single use needle sleeve which affixes to the clampstand and guides the biopsy needle to the targeted site.
“We are pleased to be able to offer this new technology to our patients, and further advance the robust offerings of our oncology program,” said Sheri Montgomery, chief executive officer for Good Samaritan Medical Center. “After a cancer diagnosis, it is important for patients to be able to have access to the latest treatment options close to home, and we are committed to offering those kinds of services at Good Sam.”
Prostate cancer is common among American men. The chances of getting prostate cancer may be affected by your age, race, family history and diet.