“Both in clinical trials and real-world clinical practice, we have seen that the use of the HeartFlow Analysis in a coronary diagnostic pathway delivers a significant reduction in invasive angiograms which are in retrospect unnecessary,” said Campbell Rogers, MD, FACC, Chief Medical Officer, HeartFlow.
HeartFlow Announces Enrollment of First Three Patients in FUSION Trial
Linkedin
Twitter
WhatsApp
Facebook
Copy Link
HeartFlow, Inc., the leader in revolutionizing precision heart care, today announced that physicians at Erasmus MC Hospital (Rotterdam NL) have enrolled the first three patients in the FUSION (Addition of FFRct in the diagnostic pathway of patients with stable chest pain to reduce unnecessary invasive coronary angiography) randomized controlled trial.
The FUSION trial, which is supported by the National Health Care Institute of the Netherlands (subsidieregeling Veelbelovende zorg: Zorginstituut Nederland / ZonMw), will evaluate whether the use of the HeartFlow FFRct Analysis as part of a coronary diagnostic pathway is effective in reducing unnecessary invasive coronary angiograms (ICAs).
Each year, 180,000 patients in the Netherlands visit a cardiologist with complaints of chest pain.1 As part of the diagnostic pathway, patients typically receive a coronary computed tomography angiogram (CTA) to determine if there is a stenosis or narrowing in the coronary artery. The degree to which a narrowing is causing chest pain is not always clear from a coronary CTA alone and patients are often sent for an ICA. Over half of patients who undergo an ICA are found to have no coronary disease or only non-obstructive disease2, making the ICA unnecessary in retrospect.
“By combining the functional information provided by HeartFlow FFRct with the anatomical information from the coronary CTA, we will be better able to assess non-invasively which patients require further invasive investigation,” said Ricardo Budde, MD, Ph.D., Associate Professor and Principal Investigator of Cardiovascular Imaging, Erasmus MC Hospital. “With the FUSION trial, we anticipate a 33% reduction in unnecessary ICAs which we believe will lead to cost savings for the overall healthcare system.”
The FUSION trial is planned to enroll 528 patients from six Dutch hospitals including Erasmus MC, UMCG, UMC Utrecht, the Admiraal de Ruyter Hospital in Goes, St Jansdal in Lelystad and Gelre hospitals Apeldoorn. Patients whose CTA shows coronary artery disease will be randomized between the HeartFlow FFRct arm or the ICA arm. The primary endpoint is the rate of unnecessary ICAs as reflected by ICAs without an obstructive coronary stenosis defined as an anatomical narrowing >50% or invasive FFR ≤0.80. Once the trial is complete, it is anticipated that the data will support insurance reimbursement in the Netherlands for the HeartFlow Analysis.
“Both in clinical trials and real-world clinical practice, we have seen that the use of the HeartFlow Analysis in a coronary diagnostic pathway delivers a significant reduction in invasive angiograms which are in retrospect unnecessary,” said Campbell Rogers, MD, FACC, Chief Medical Officer, HeartFlow. “We anticipate the FUSION trial will deliver similar outcomes to our previous studies and look forward to working with the National Health Care Institute to make the HeartFlow Analysis available to patients in the Netherlands in the near future.”
About the HeartFlow FFRct Analysis
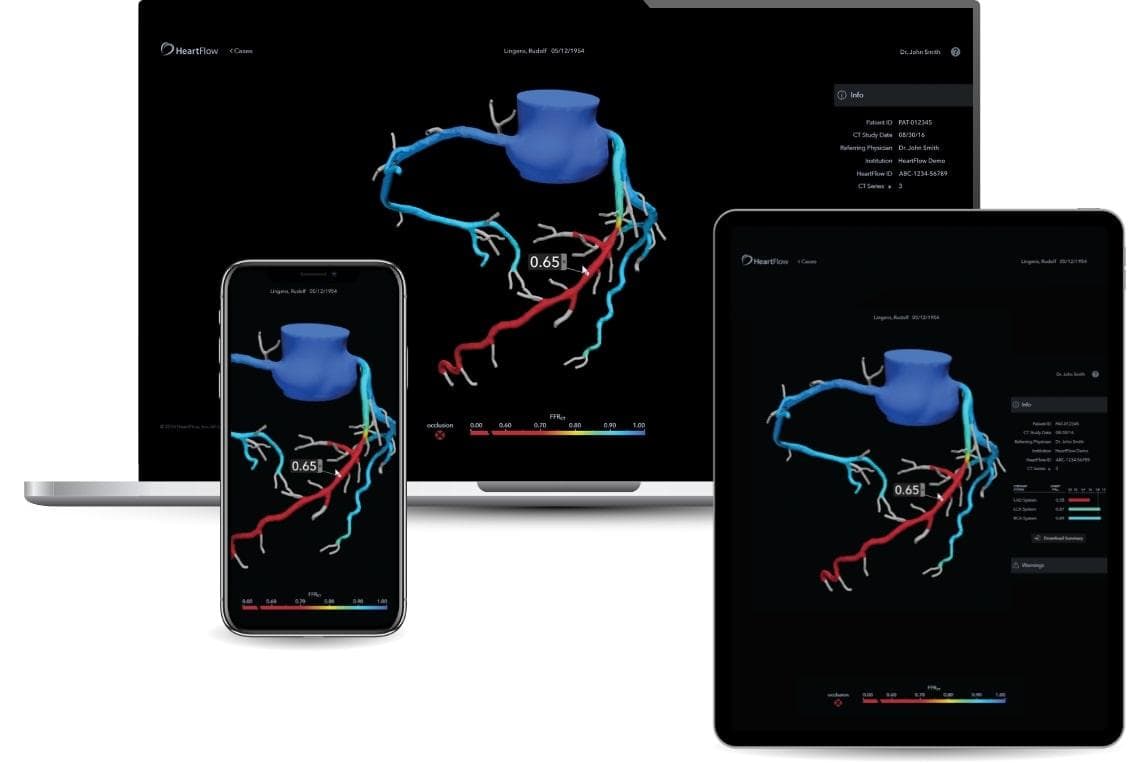
Starting with a standard coronary computed tomography angiogram (CTA), the HeartFlow Analysis leverages deep learning and highly trained analysts to create a digital, personalized 3D model of the heart. The HeartFlow Analysis then uses powerful computer algorithms to solve millions of complex equations to simulate blood flow and provides FFRct values along the coronary arteries. This information helps physicians evaluate the impact a blockage may be having on blood flow and determine the optimal course of treatment for each patient. A positive FFRct value (≤0.80) indicates that a coronary blockage is impeding blood flow to the heart muscle to a degree which may warrant invasive management.
Data demonstrating the safety, efficacy and cost-effectiveness of the HeartFlow Analysis have been published in more than 425 peer-reviewed publications, including long-term data out to five years. The HeartFlow Analysis offers the highest diagnostic performance available from a non-invasive test.3 To date, clinicians around the world have used the HeartFlow Analysis for more than 100,000 patients to aid in the diagnosis of heart disease.