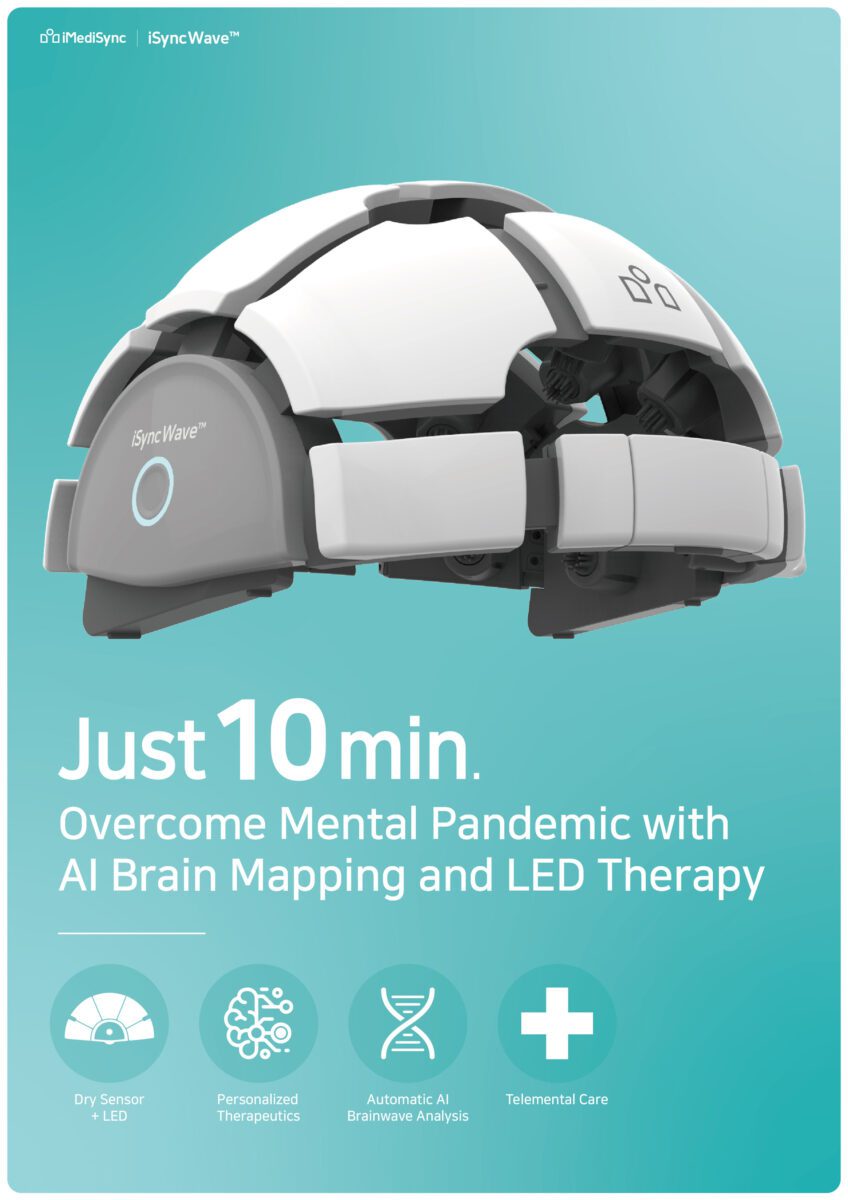
iMediSync, a leading AI-driven early detection and therapeutic platform for optimal brain health from Seoul, South Korea announced today that the company will showcase its comprehensive EEG solution (hardware + software + remote telehealth solution), to perform brain mental health screening & predictive analysis of potential mental conditions in just 10 minutes.
They are launching their first therapeutic device – iSyncWave™, which integrates both EEG brain mapping and LED therapy at 2022 CES Las Vegas. An open-to-public CES Show Day press conference will take place on Jan 6th at the CES provided conference room.
iSyncWave™ is an innovative brain-scanning device with high-quality dry EEG sensors that autofit to various sizes and shapes, keeping 10 to 20 system locations and equipped with matching sex/age Norm DB.
As the company slogan states, “Overcome mental pandemic in just 10 minutes, “press and visitors can expect a complete analysis report of EEG (brainwave) and HRV (Heart rate variability) operated by AI deep learning algorithms in just 10 minutes to assess the condition of brain and dysfunctionality including the early diagnostic insights which could play a major role in discovering a neuro-related diseases in early stages.
Recently with the addition of software upgrades, and an FCC approval allowed the iSyncWave to be exported to the EU, Asia, and Australia already use for research and diagnostics.
“We will provide life-changing mental healthcare experience to introduce neuro-mental illness biomarker and the patent EEG/HRV technology,” says CEO Dr. Seung Wan Kang. “iMediSync takes its leap to be a trend leader in the field of telemedicine, and AI medicine, digital therapy will take place in a post-Covid-19 era as an optimized individual mental healthcare service provider.”
iMediSync proposes an EEG-centric global vision of integrated telemental health care & CNS drug development platform while cooperating with various neurologists, hospitals in South Korea in the field of Clinical Depression, Parkinson’s Disease, and other neuro-related diseases to expand the biomarker R&D pipeline. Active communication with global pharmaceutical companies are ongoing as well.