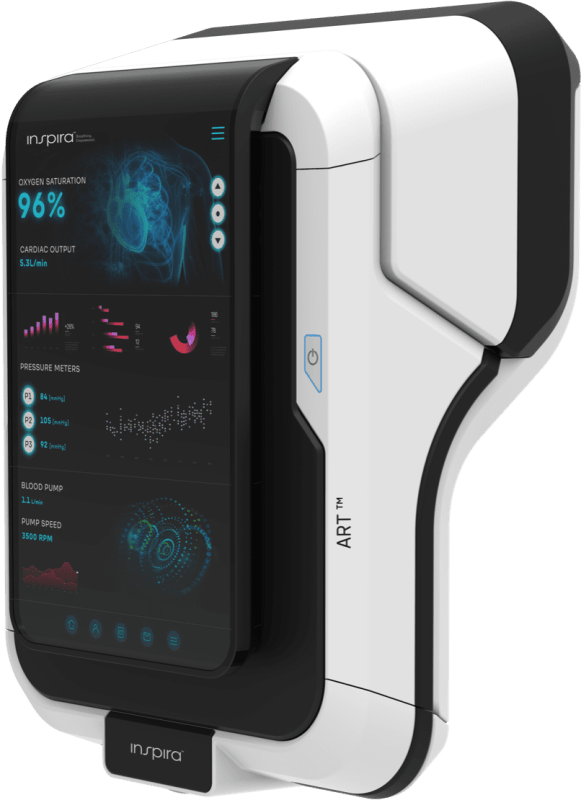
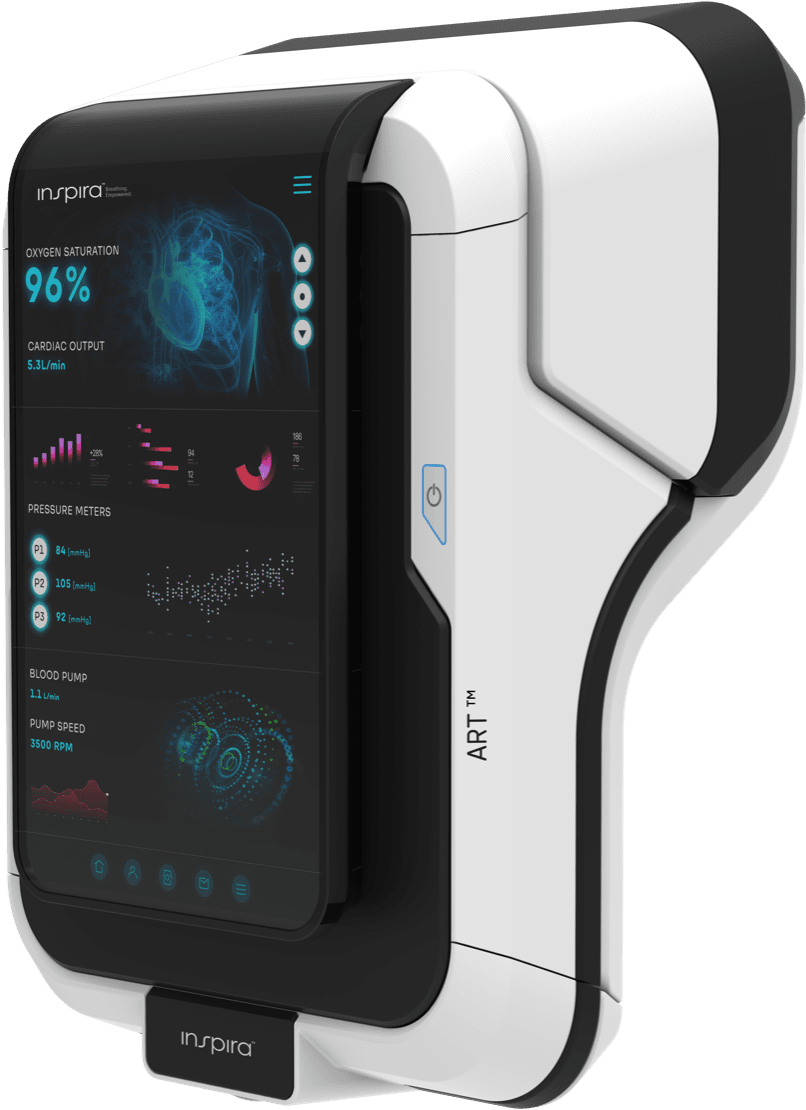
Inspira Technologies OXY B.H.N. Ltd. (Nasdaq: IINN, IINNW) (the “Company” or “Inspira”), a breakthrough medical technology company, today announced that is has received the first purchase order from Glo-Med Networks, Inc. (“Glo-Med”) for its INSPIRA™ ART100 systems, with the five product units expected to be shipped in the fourth quarter of 2024. The purchase order also provides for the potential purchase of an additional 20 systems, the timing of which is subject to further agreement between Inspira and Glo-Med.
The planned shipment and potential additional sales of the INSPIRA™ ART100 systems is a pivotal step in the Company’s growth model, which is primarily based on the INSPIRA™ ART (Gen 2) that is currently in development and is expected to target the $19 billion mechanical ventilator market, with its next generation cutting-edge technology designed to replace the need for mechanical ventilation. The Company plans to offer hospitals receiving the INSPIRA™ ART100 an option with special terms for the integrated HYLA™ Blood Sensor and INSPIRA™ ART (Gen 2) devices, subject to the completion of the development of those products, which are also subject to approval by regulatory entities.
Joe Hayon, Director, President and co-founder of Inspira stated: “We continue to develop the breakthrough technologies of tomorrow. The INSPIRA™ ART100, is our first and important step in building the INSPIRA market presence and position in preparation for the INSPIRA™ ART (Gen 2), which we believe has the potential to provide a new clinical solution with a potentially different intent of use for the mega life support and mechanical ventilation market.”
The Company already began the commercial manufacturing of the INSPIRA™ ART100 with the first five units planned for shipment to Glo-Med, a distributor for Inspira products in the U.S., with the devices planned for deployment in U.S. hospitals.
Inspira received FDA 510(k) clearance for its INSPIRA ART100, a Cardiopulmonary Bypass System.
The Company’s other products, including the INSPIRA ART (Gen 2) and HYLA™ blood sensor, have not yet been tested or used in humans and have not been approved by any regulatory entity.