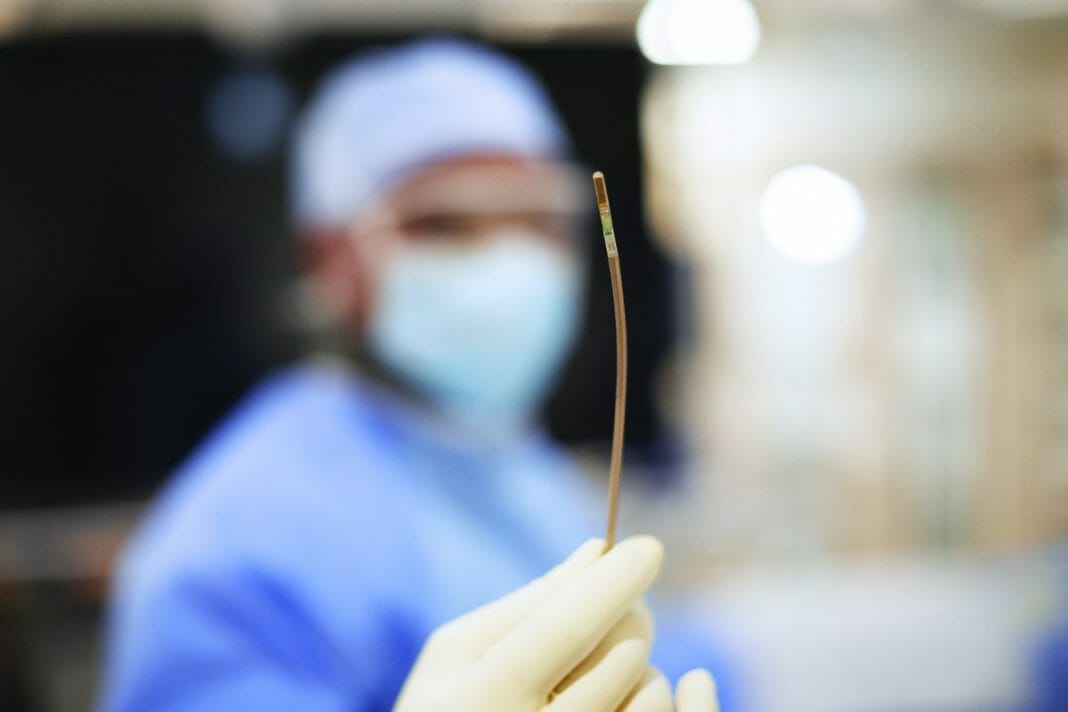
Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced that Baptist Health’s Miami Cardiac & Vascular Institute (Florida, U.S.) has successfully started treating patients with Verisight Pro, Philips’ real-time 3D Intracardiac Echocardiography (ICE) catheter, during minimally-invasive image-guided procedures for structural heart disease.
Offering superior 2D and 3D live imaging, VeriSight Pro allows interventionalists to navigate procedures with ease, provide superior care, and optimize performance of the interventional suite to benefit more heart patients. With Philips’ intracardiac echocardiography (ICE) catheter VeriSight Pro, additional groups of patients can now be treated, such as those who are not suitable for transesophageal echocardiography (TEE) [1], which typically requires heavy sedation or general anesthesia.
Dr. Ramon Quesada and Dr. Bernardo Lopez-Sanabria are the first two interventional cardiologists to use this technology for Baptist Health.
Dr. Ramon Quesada, interventional cardiologist with Miami Cardiac & Vascular Institute said:
“VeriSight Pro’s technology is a game changer, allowing us to give people who need this procedure a chance for a smoother and safer recovery. As physicians, we are always exploring new ways to care for our patients and provide them with the best treatment options for their long-term future.”
Dr. Bernardo Lopez-Sanabria, interventional cardiologist at Miami Cardiac & Vascular Institute said:
“This device allows our physicians to capture the very best possible live images and enable our team to deliver quality outcomes for our cardiac patients, so they can get back to their daily routines. I am honored to be the first physician in South Florida to use this new technology and bring it to our community.”
Ultrasound imaging to guide structural heart repair and electrophysiology procedures has conventionally been via transthoracic echocardiography (TTE), in which an ultrasound probe is placed on the patient’s chest, or via transesophageal echocardiography (TEE), which involves the insertion of an ultrasound probe into the patient’s esophagus so that it lies close to their heart. However, TTE typically results in sub-optimal imaging, while TEE requires heavy sedation or general anesthesia so that the patient can tolerate the procedure. To overcome these limitations, Philips ICE catheter utilizes an ultra-miniature ultrasound probe mounted on the end of a 3mm diameter (9 French) catheter, allowing it to be routed via the patient’s vasculature to inside their heart – the same route used to introduce other catheters during minimally-invasive cardiac surgery. Based on Philips’ innovative xMATRIX array 3D ultrasound transducer technology, VeriSight Pro’s probe captures high-quality 2D and 3D live images of the heart, including a 90-degree by 90-degree 3D field of view, simultaneous viewing of two scan planes (for example, to measure the long and short axes of the left atrial appendage), plus 3D volume and color-flow images. Unique to Philips’ device, interventionalists can digitally change the scan angle to capture different views without having to manually reposition the ultrasound tip.
Chris Landon, Senior Vice President and General Manager Image Guided Therapy Devices at Philips said:
“The exceptional 2D and 3D imaging capabilities of the new Philips Intracardiac Echocardiology Catheter – VeriSight Pro – make it a superb choice for image guidance in interventional procedures, reducing reliance on general anesthesia and the logistical hurdles that go with it. Adoption of the technology by Baptist Health’s Miami Cardiac & Vascular Institute, one of the most innovative and top institutes in the world, is a significant milestone in bringing better heart care to more patients.”
Philips’ FDA 510(k)-cleared 3D ICE catheter, VeriSight Pro, has the potential to improve the standard of care for structural heart disease and electrophysiology procedures and broaden the range of treatment options open to patients. The reduced need for heavy sedation or general anesthesia accelerates workflows, contributing to increased cath lab efficiency and shorter patient preparation and recovery times. The catheter is designed for use with Philips’ premium cardiology ultrasound systems, EPIQ CVx and EPIQ CVxi.
Philips Intracardiac Echocardiology Catheter – VeriSight Pro – adds to the company’s comprehensive suite of integrated solutions for the treatment of structural heart disease, which combines interventional X-ray, 3D ultrasound, and related interventional tools to enable confident navigation and positioning of devices to optimize outcomes and reduce procedure times.
[1] A transesophageal echocardiogram is done by inserting a probe with a transducer down the esophagus