October 13, 2020
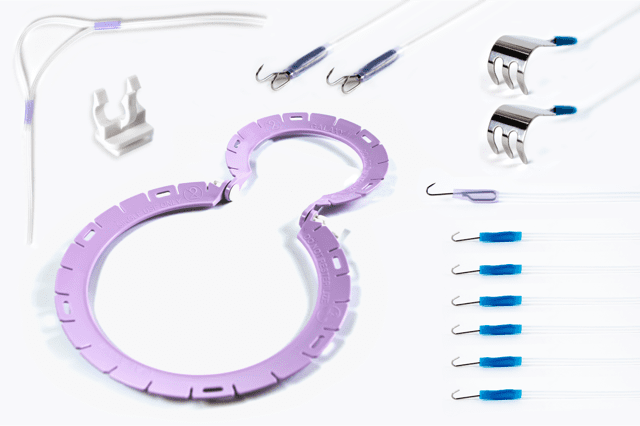
JUNE Medical is expanding its comprehensive portfolio of medical devices with the upcoming launch of its new pre-packed retractor kits, especially for male procedures. Based on the company’s award-winning Galaxy II retractor, the Galaxy II Male kit brings the same level of innovation to male surgeries.
June Medical notes the Galaxy II Male kit is set to revolutionize male procedures by dramatically improving visualization during surgery. This straightforward and easy to use kit is suitable for a wide range of procedures requiring optimal control over the surgical site, such as scrotal and associated surgeries, as well as prosthetic testicle implantations. The kit features a number of different accessories and a novel hammock elevation strap to maneuver the male anatomy, allowing the penis to be held securely without requiring hooks through the skin, improving patient comfort.
The kit also benefits from all of the characteristics that have made the Galaxy II system so popular. The retractor’s unique, design-protected cam locks allow simple single-handed adjustment for rapid repositioning, while the slots on the retractor ring easily secure the stays in place. Together with a range of stay hooks designed to enable high tension without the risk of tissue tearing, this system provides an innovative, cost-effective, and convenient solution for a wide range of surgical procedures.
Angela Spang, CEO of JUNE Medical, commented: “The Galaxy II frees up the hands of assistants and nurses that often have to hold the retractor, sometimes for long periods of time. This improves efficiency and reduces the number of people needed in the operating room, which is especially useful during the COVID-19 pandemic to help reduce infection risk.”