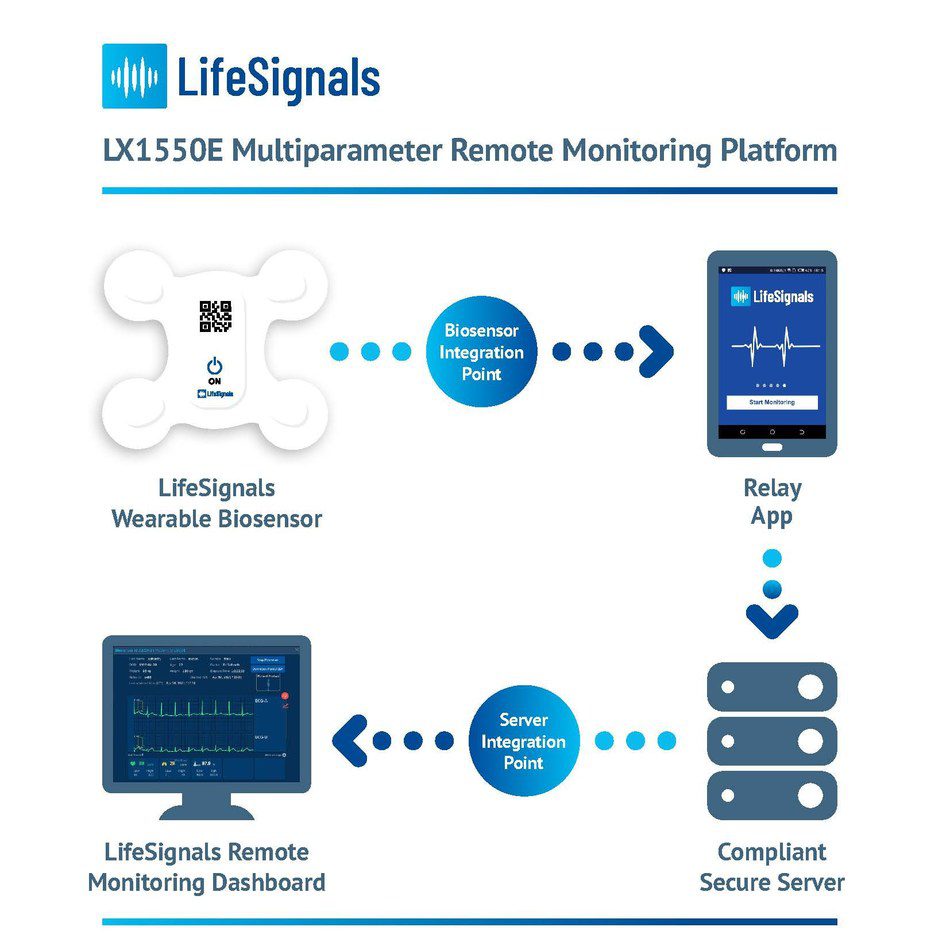
LifeSignals Inc., today announced the CE (Conformité Européene) Mark for the LifeSignals LX1550E Multiparameter Remote Monitoring Platform – which can be used by clinicians for the continuous collection of physiological data of patients at home and in healthcare settings.
The single-use, wearable medical biosensor, records Electrocardiography (2-channel ECG), Heart Rate, Respiration rate, Skin Temperature and Body Posture data for up to five days. The encrypted physiological data can then be transmitted with high reliability from the LifeSignals Biosensor, via the Relay App to a secure cloud-based platform. Clinicians and Care Providers can access the cloud-based LifeSignals Remote Monitoring Dashboard to view patient physiological data and manage vital sign alert settings.
The LifeSignals Remote Monitoring Platform is designed to enable healthtech companies to rapidly enhance their product and service portfolios to provide vital sign monitoring to the widest possible patient base, from any location. The interoperable platform can be tailored with ready-to-deploy software development kit APIs and is suitable for large scale implementation.
“COVID-19 has accelerated the adoption of remote patient monitoring globally,” says Steve Jones, Business Development Director, Europe. “This regulatory approval for our Multiparameter Platform is a significant breakthrough and will enable our Partners to bring to market affordable and scalable remote monitoring products and solutions”.