An increasing number of physicians have been utilizing a unique gas exchange analyzer technology to enhance the recognition and diagnosis of pulmonary embolism (PE) in patients.
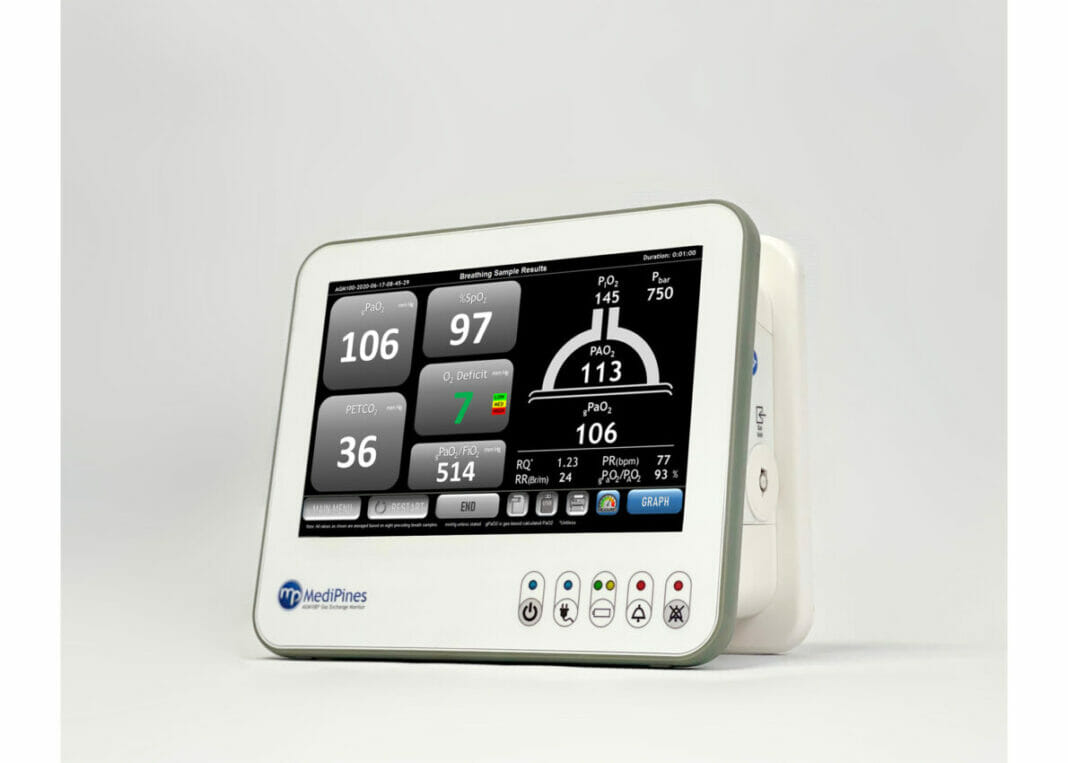
The device, called the MediPines AGM100, is an FDA-cleared advanced pulmonary gas exchange technology that can precisely and non-invasively measure patients’ oxygen and carbon dioxide levels in the deepest part of the lungs (alveoli), as well as oxygen deficit (alveolar-arterial difference), which support assessment of respiratory impairment.
A case study titled “Use of a Non-Invasive Pulmonary Gas-Exchange Analyzer to Improve the Pretest Probability of Pulmonary Embolism in a Patient Classified as ‘Low Risk’” by Dylan Sieck Ph.D. and Pierre Ozon, MD was presented at the American Thoracic Society (ATS) International Conference last year. Since then, more and more clinicians and centers of excellence have been turning to the MediPines AGM100 as a diagnostic support tool to enhance recognition of PE.
PE is one of the leading causes of death in the US and the third leading cause of death in hospitalized patients. Given the possibility of asymptomatic and atypical presentation, it is generally accepted that many cases of PE go undiagnosed.
In the case study presented at ATS last year, the MediPines AGM100 device, a cardiopulmonary diagnostic support system, was shown to be a helpful adjunct tool in the diagnosis of PE and ideally suited to the task because of the quick and precise measurements it provides. The patient described in the case study was the first reported case of a non-invasive pulmonary gas-exchange analysis being used to improve pre-test probability of PE. Since then, more physicians have been turning to the technology as a diagnostic tool.
“Having an objective measure of pulmonary gas exchange impairment can factor into the diagnosis and treatment decisions that must be made in a time sensitive manner,” said Dr. Pirre Ozon, an Emergency Physician, and one of the case study’s authors. “The AGM100’s multiple measurements including oxygen deficit measurement can be highly informative in narrowing differential diagnosis scenarios similar to this case and more research is needed to fully understand its potential.”
Designated by the World Health Organization (WHO) as one of 15 notably innovative and commercially available health technologies for the treatment of COVID-19 and other global priority diseases in its 2021 edition of the WHO Compendium of Innovative Health Technologies, the MediPines AGM100 is a portable system that can be used throughout hospitals or qualified health clinics