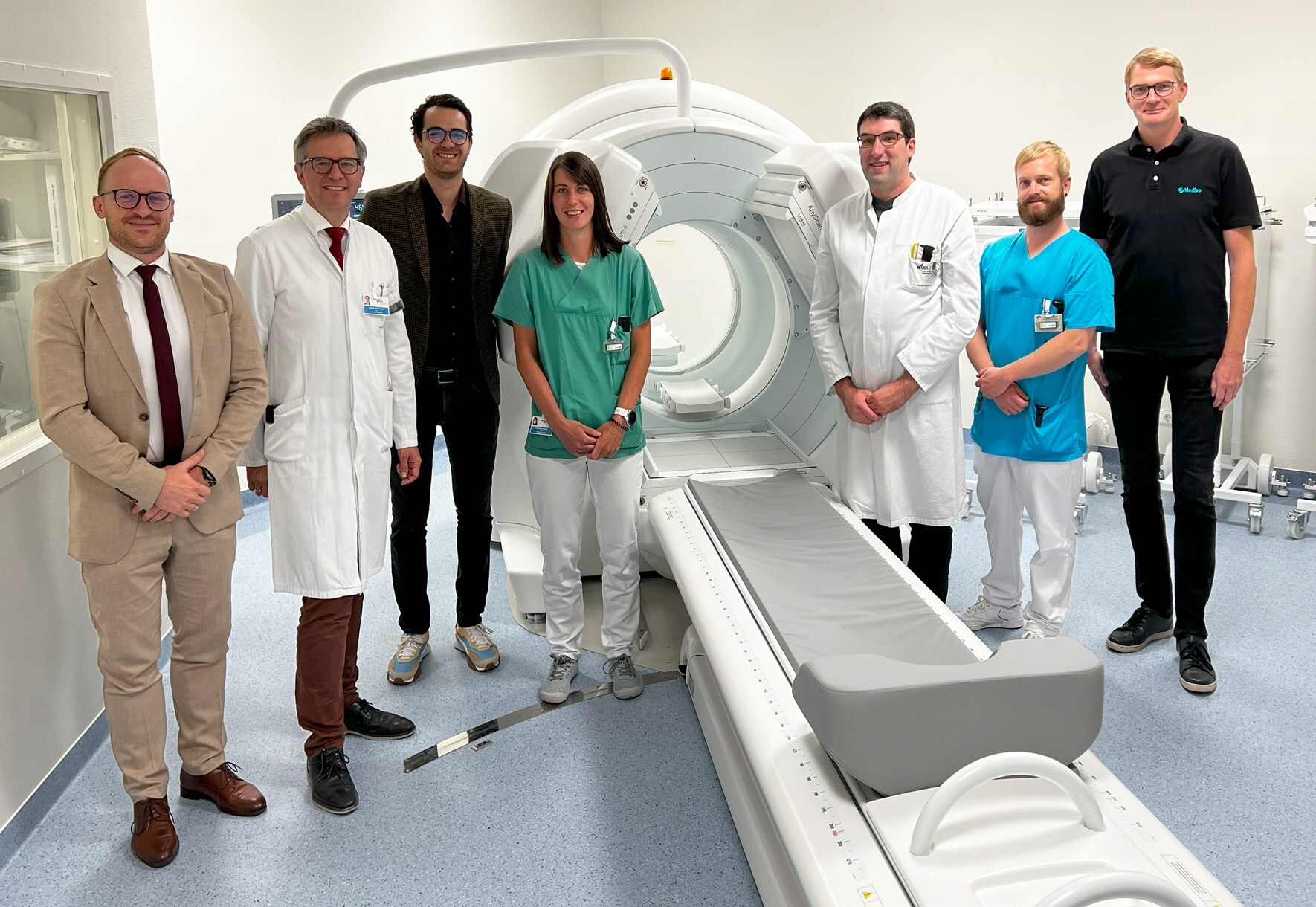
Mediso is unveiling its new AnyScan® TRIO SPECT/CT, TheraMAX * scanner at the annual meeting of European Association of Nuclear Medicine (EANM).
The new TheraMAX is specifically designed for serving the clinical needs of theranostic imaging. It features detectors and collimators developed for Targeted Radionuclide Therapies carried out with radioisotopes emitting not only alpha and beta particles, but high and ultra-high energy gamma photons like 177Lu, 212Pb, 131I, 225Ac and beyond.
The TheraMAX is equipped with three, large surface detectors (up to 5x larger than 12-detector CZT systems) surrounding the patients completely (360°). The 15.9mm thick NaI crystals ensure improved sensitivity for high energy photons, while the high-density sensor arrangement (123 PMT per detector head) allows high spatial resolution leading to PET-like image quality in all NM applications.
The 300% sensitivity gain compared to conventional dual-detector systems enables whole body 225Ac SPECT/CT in theranostic imaging, while this extreme total system response also allowing ultra-fast quantitative total-body SPECT/CT scans with 99mTc and LEHR-HS collimator. Moreover, novel multi-pinhole technology combined with Triple-NaI-Detectors provide outstanding performance for cardiology and neurology.
The integrated CT offers three performance levels: low-dose diagnostic scans, ultra-low dose protocol for attenuation and scatter correction and even “Zero-Dose” imaging with AI-powered SyCT** (Synthetic CT).
The first global site for the TheraMAX is the University Hospital Regensburg, Germany. Following a recent installation, Professor Dr. Dirk Hellwig, the head of the Department of Nuclear Medicine said “the new scanner will boost our diagnostic and theranostic workflow, leading to better patient care and it also opens new possibilities in clinical research”.
“With the development of the new TheraMAX, we extended the imaging capabilities of nuclear medicine departments by improving the diagnostic confidence of SPECT/CT examinations close to PET/CT” said Istvan Bagaméry, Managing Director of Mediso Ltd. “The TheraMAX transforms the clinical routine also with ultra-fast quantitative total-body scans.”