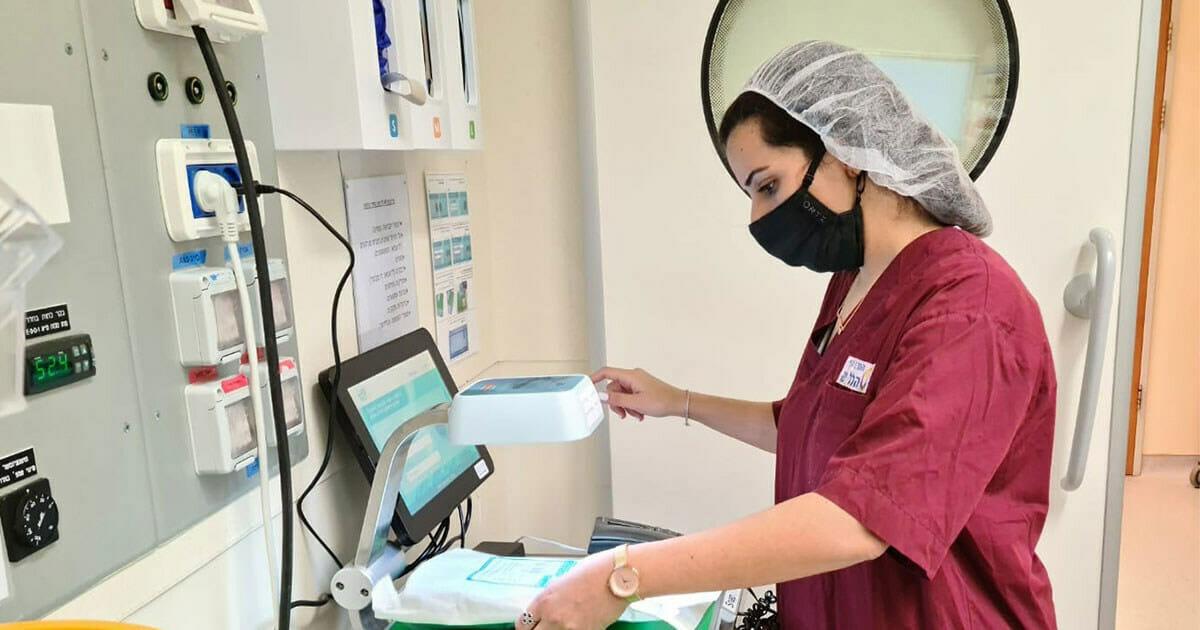
A dynamic and global leader in point-of-use data sensing solutions, IDENTI Medical designs, develops and produces advanced data collection solutions for surgery, procedure and clean supply room settings.

Backed by $2 Million, Droplet IV is Set to Launch its Revolutionary Automatic IV-Line Flushing Device in EU & US in 2026
Droplet IV today announced the successful completion of a $2 million funding round to enable launch of its first automatic IV-line flushing device in the