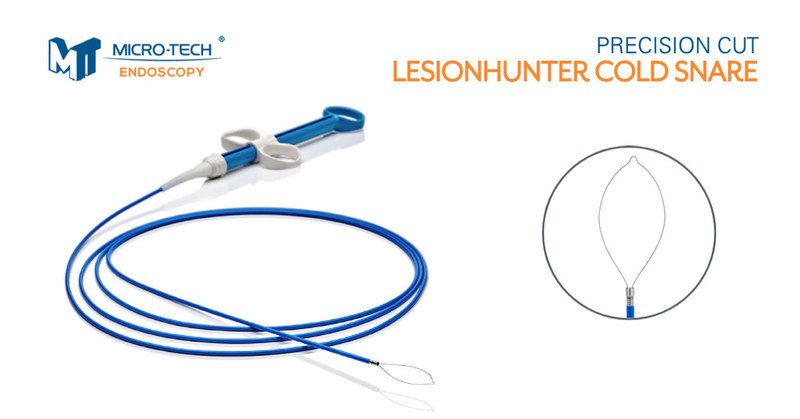
Micro-Tech Endoscopy launched the LesionHunter Cold Snare, a novel cold snare with an ultra-thin Nitinol wire. Key features include:
- Thin Monofilament Nitinol Wire – this wire material provides durability in the shape and allows for a 0.18mm thin wire for a clean and precise cut.
- Coiled Sheath – the coiled metal catheter creates greater stability for the guillotine-like cutting action of the wire.
- Reduced Fly-Away Tissue – the thin wire may reduce the risk of generating a “fly-away” specimen; thereby making it easier to find the tissue at the end of the resection.
“The LesionHunter is a unique cold snare with two great features, the ability to trap the tissue regardless of how flat the lesion is, and the ‘cheese cutter’ capability to resect even larger lesions,” commented Gastroenterologist and Advanced Endoscopist at NYU Langone, Gregory Haber, M.D. “It has become my go-to for cold snare applications.”
The LesionHunter nitinol snare was specifically designed to advance cold snare polypectomy.
“The LesionHunter nitinol wire is 61% thinner than traditional braided snare wires and 42% thinner than the most popular braided cold snare on the market,” Micro-Tech’s Executive Global R&D Director Scott Haack said. “The thin Nitinol wire allows for multiple tissue resections while maintaining its shape along with the ability to make a clean cut through large pieces of tissue. Current dedicated cold snares and hybrid hot/cold snares can’t consistently accomplish this.”