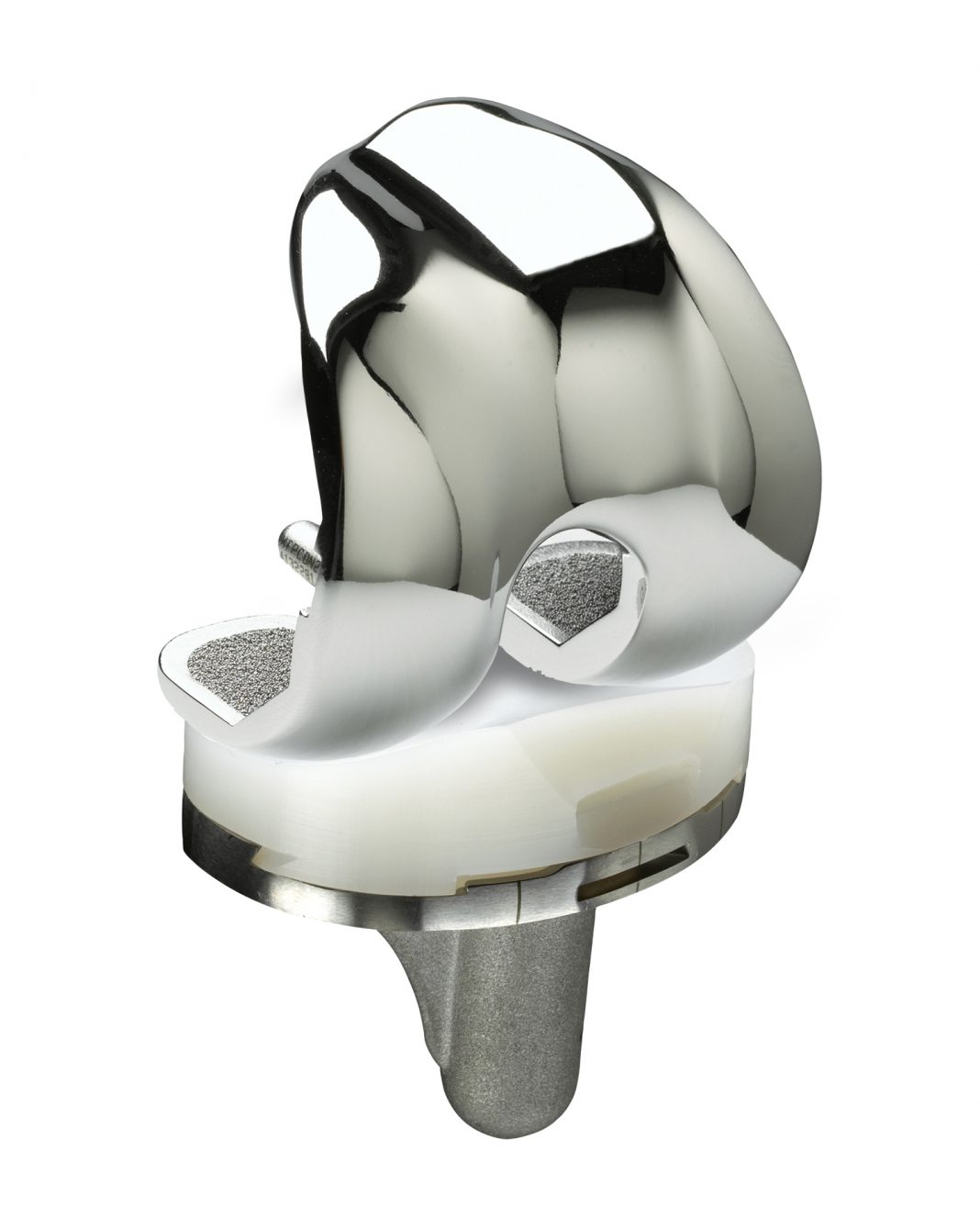
MicroPort Orthopedics, a global leader in orthopedic devices and technologies, has received a “15A” rating for its Advance® Medial-Pivot knee system from the Orthopaedic Data Evaluation Panel (ODEP).
MicroPort Orthopedics’ medial-pivot knee systems stand on more than 20 years of clinically demonstrated history with a complete portfolio of medial-pivot design knees. The Advance® Medial-Pivot knee system was built on the latest kinematic evidence of the natural stability and motion of the knee.1 Its innovative design is carried on by MicroPort’s latest medial-pivot knee, the Evolution® Medial-Pivot knee system, which also aims to restore function by replicating natural motion and stability, and to address some of the common problems seen with traditional designs, such as anterior knee pain and quadriceps avoidance.
REFERENCES
1. George A. Macheras et al. “A long term clinical outcome of the Medial Pivot Knee Arthroplasty System.” The Knee Journal, January 29, 2017.
2. Patient satisfaction at 2 months following total knee replacement using a second generation medial-pivot system: follow-up of 250 consecutive cases. Van Overschelde P et al. Ann Transl Med 2016
3. Pritchett JW. Patients prefer a bicruciate-retaining or the medial pivot total knee prosthesis. J Arthroplasty. 2011;26:224–228.
4. Miyazaki Y et al. Analysis of the Kinematics of Total Knee Prostheses With a Medial Pivot Design. J Arthroplasty. 2011; 26(7):1038-1044.
5. Lamontagne M et al.. Muscle Activity in Total Knee Arthroplasty Patients While Ascending and Descending a Ramp. Orthopaedic Proceedings, 2019. 101-B(SUPP_5): p. 59-59.