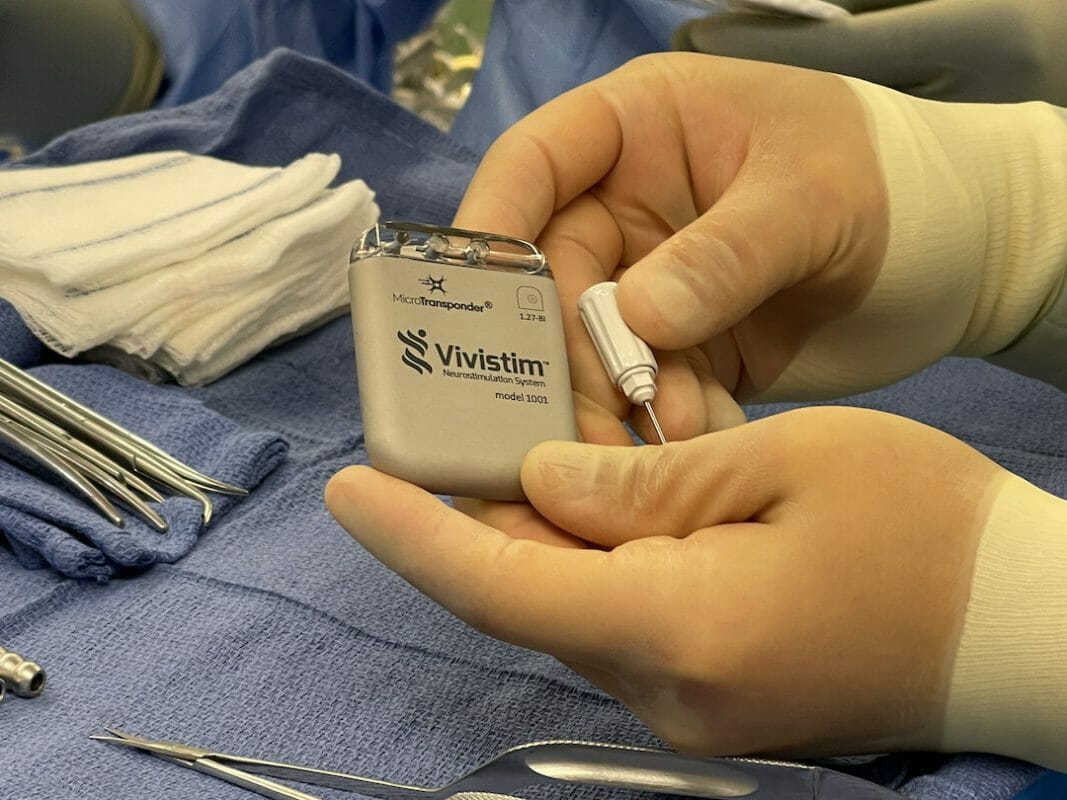
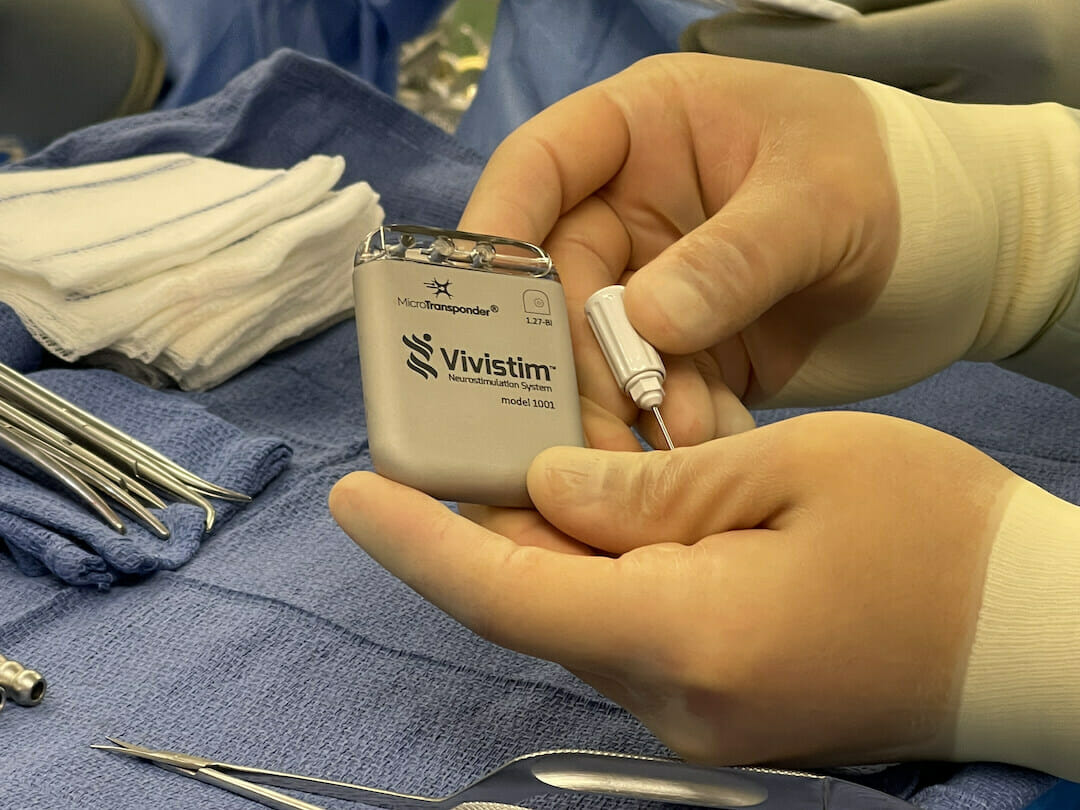
MicroTransponder ®, Inc. announces that the Centers for Medicare and Medicaid Services (CMS) awarded the Vivistim® Paired VNS™ System transitional pass-through status, expanding access to Medicare beneficiaries who are ischemic stroke survivors interested in using the breakthrough technology to help improve their hand and arm mobility.
The Vivistim Paired VNS System is an FDA Breakthrough Device that was granted pre-market authorization (PMA) approval on August 27, 2021. Unlike other neurostimulation technologies, the Vivistim System is used in conjunction with rehabilitation therapy to help ischemic stroke survivors who have chronic arm and hand deficits improve their upper limb function. Enhancing the effectiveness of rehabilitation therapy, Paired VNS Therapy is clinically proven to generate two to three times more hand and arm function than rehabilitation alone.
To benefit from the system, ischemic stroke survivors will have the small Vivistim device implanted in their upper left chest area during an outpatient procedure. Once cleared for rehabilitation, they engage in physical or occupational therapy where a therapist uses a wireless transmitter to signal the Vivistim device to deliver a gentle pulse to the vagus nerve while the stroke survivor performs a specific task, such as putting on a hat, brushing hair or cutting food. The simultaneous pairing of the rehabilitation exercise in high repetitions with vagus nerve stimulation helps increase neuroplasticity and strengthens neural connections to improve upper limb function.
“Hospitals treating stroke survivors with the Vivistim System are now eligible for additional reimbursement through Medicare’s outpatient prospective payment system,” said Richard Foust, MicroTransponder’s CEO, adding that the new CMS payment status for Vivistim became effective January 1, 2023. “As a result, there’s now another pathway for stroke survivors to benefit from Paired VNS Therapy at the recommendation of their healthcare providers.”
Neurological and rehabilitation specialists at premier comprehensive stroke centers and rehabilitation centers report that the Vivistim Paired VNS System helps ischemic stroke survivors improve their quality of life by increasing their ability to perform activities of daily living.
For instance, Rosa Maria Villalpando, the first stroke survivor to use the Vivistim System commercially, was paralyzed on her left side after her stroke in 2017. She credits the Vivistim Therapy she received at Keck Medicine of USC for helping her to be able to open her left hand and relax her left arm. Similarly, David Sullivan has struggled to use his left hand and arm since surviving a stroke in 2020. His healthcare team at Massachusetts General Hospital and Spaulding Rehabilitation Hospital recommended Vivistim, which has helped him make gains, such as swinging a bat.
“The life-changing clinical outcomes of Vivistim Paired VNS Therapy that are already being reported across the country affirm CMS’ determination that the Vivistim System is novel and represents a substantial clinical improvement over existing technologies,” said Doug Ellison, MicroTransponder’s chief revenue officer. “As we advance Vivistim commercialization, CMS’ pass-through status enables providers to realize the clinical, operational and financial benefits of adopting Vivistim as their standard of care for chronic ischemic stroke rehabilitation.”