Monteris Medical, the leader in MRI-guided laser interstitial thermotherapy (LITT or laser ablation), announced today that health insurance provider Health Care Service Corporation (HCSC), one of the largest independent licensees of Blue Cross and Blue Shield Association® (BCBS), has updated its medical coverage policy allowing access to the minimally invasive laser ablation procedure for brain tumors and epilepsy when medically necessary.
Monteris Medical advises the updated policy covers 16 million lives in the states of Illinois, Texas, Oklahoma, New Mexico and Montana.
This update follows a similar policy decision by Blue Cross Blue Shield of North Carolina, which also provides coverage of MRI-guided laser ablation for patients with brain tumors and epilepsy. A growing body of peer-reviewed clinical evidence continues to show medical advantages of MRI-guided laser ablation technology over craniotomy for resection due to decreased morbidity from a minimally invasive procedure, faster recovery time, shorter hospital and intensive care stay, and an ability to access lesions not amenable to surgery. Laser ablation also represents an alternative to surgery for patients with significant comorbidities.
“The implications of this policy coverage addition by HCSC are significant for the patients we see every day who are not candidates for a craniotomy for treatment of a primary or metastatic brain tumor or radiation necrosis,” said Dr. Ganesh Rao, professor and chairman of the Department of Neurosurgery at Baylor College of Medicine in Houston, Texas. “This decision will provide our patients with greater access to minimally invasive technology, which can positively impact their care and improve their quality of life.”
Many patients with brain tumors face situations in which traditional open brain surgery is neither possible nor the preferred approach. In addition, many drug-resistant epilepsy patients may be candidates for surgical intervention when their seizures are not controlled with medication.
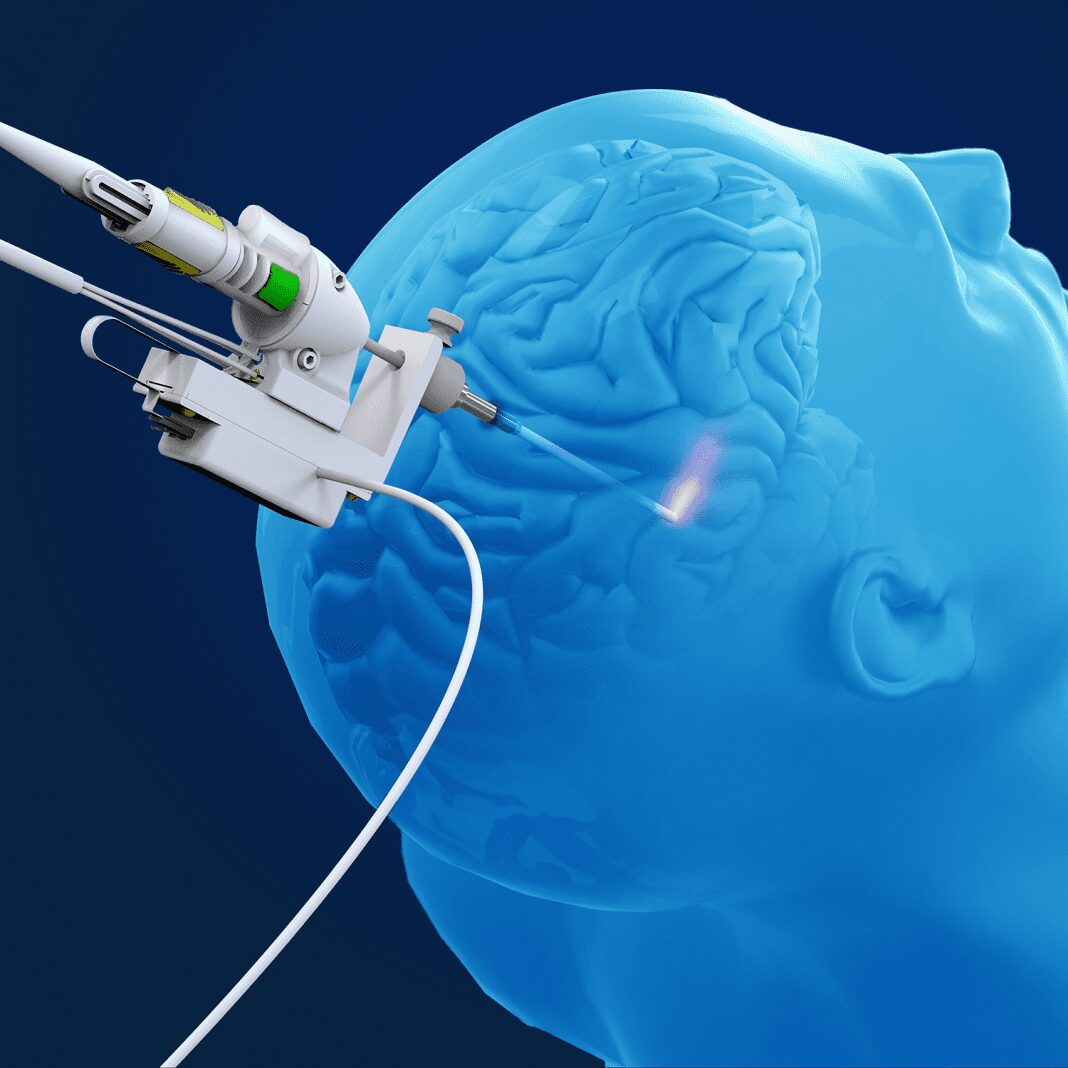
The Monteris Medical NeuroBlate laser ablation system is a tool used to destroy targeted tissue in the brain and provides a minimally invasive alternative for these patients and their physicians. Laser ablation offers the ability to target the destruction of the identified tissue while often shortening time in the hospital, reducing utilization of intensive care units, and improving the patient’s recovery from the procedure.
“All top-notch epilepsy and tumor programs need to have laser interstitial therapy in their toolkit to treat patients who don’t have other options,” said Dr. Bradley Lega, assistant professor in the Department of Neurosurgery at the University of Texas Southwestern Medical Center in Dallas, Texas. “This policy action by HCSC will allow us to serve BCBS patients with this procedure that has established benefits.”
“We are pleased that HCSC has recognized both the clinical need for patients and the resource value to hospitals that minimally invasive laser ablation offers,” said Martin J. Emerson, president and chief executive officer of Monteris Medical. “The HCSC decision comes on the heels of the American Medical Association’s application acceptance for new Category Level I CPT® codes and earlier coverage wins by Aetna®, Anthem, Inc. and Blue Cross Blue Shield of North Carolina. This series of policy successes clearly acknowledges laser ablation for the brain as an established procedure that can benefit people suffering from tumors and uncontrolled seizures.”