Esaote, located in Genoa, Italy mission is to obtain extremely clear, easy-to-read ultrasound images to ensure accurate diagnosis in the shortest time possible, enhancing the users' experience across several clinical applications.
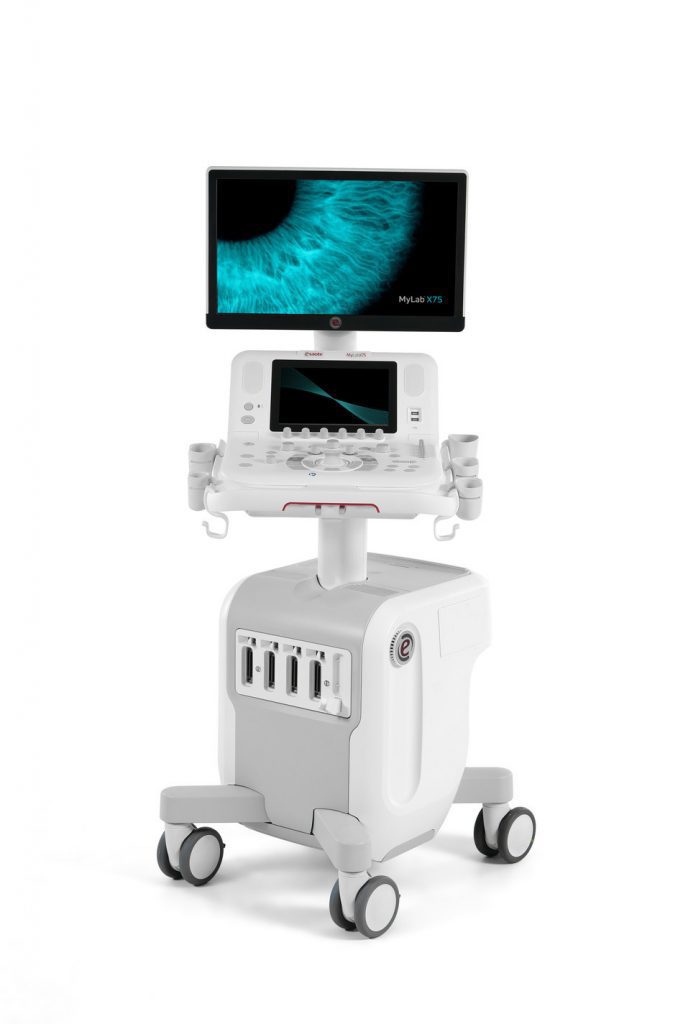
MyLab X75 Ultrasound System Launches Reports Esaote: Enriched with Unparalleled Connectivity and Image Sharing Tools
Linkedin
Twitter
WhatsApp
Facebook
Copy Link
MyLab X75 offers access to specific clinical solutions such as micro-vascularization assessment, liver stiffness quantification as well as zero-click left ventricle function analysis to always deliver the best care possible.
Esaote, located in Genoa, Italy mission is to obtain extremely clear, easy-to-read ultrasound images to ensure accurate diagnosis in the shortest time possible, enhancing the users’ experience across several clinical applications.
“In the world of imaging and non-invasive diagnostics innovation is key,” says Eugenio Biglieri, Esaote COO. “Particularly now that health organizations are under great pressure, ultrasound imaging can give a very important contribution, due to non-ionizing-radiation, agile and point-of-care approach, which ensure accessibility. In this scenario ease of use and automation are the main drivers for the technology’s adoption.”
MyLab X75 is enriched with unparalleled connectivity and image sharing tools, which clearly facilitate the system management in this challenging pandemic period and allow innovative models to be implemented.
“Voice of the customers and consolidated experience were at the core of MyLab X75’s development,” says Giovanni Altobelli, Global Marketing Product Manager, “and making ultrasound imaging easier was our first priority: light and agile, ergonomic and silent, productive and eco-friendly, the new system perfectly meets nowadays clinical needs.”
MyLab X75 is being unveiled on Friday, May 28, 2021 with an exclusive streaming event on several platforms. The event, part of the Esaote’s “Exploring the inside” program, is dedicated to the theme of Experience. Distinguished guests such as the professional alpinist Hervé Barmasse and emeritus medical experts participate in and contribute to the event.
