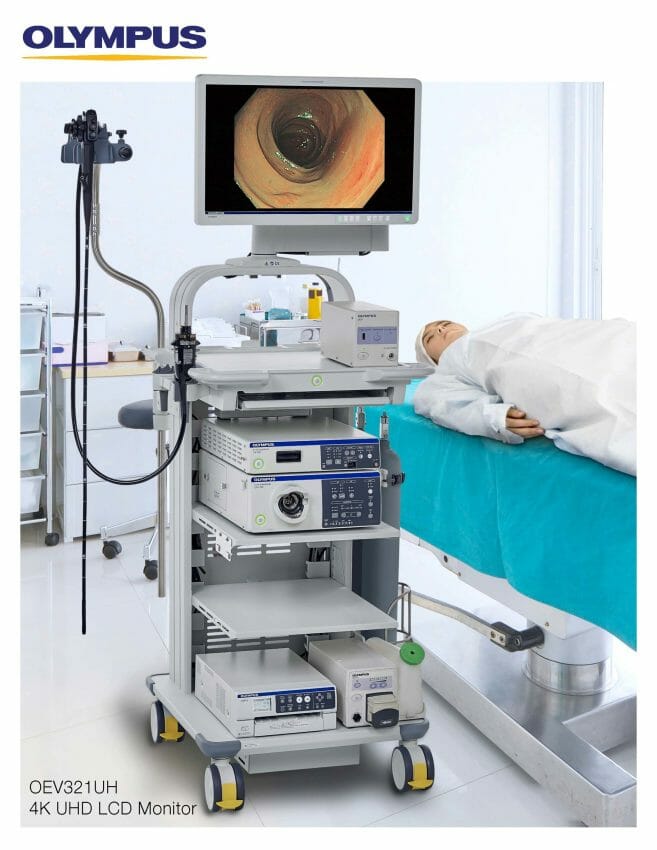
Olympus announced today the availability of a new monitor option, the OEV321UH video display monitor, a 32-inch Ultra High Definition 4K monitor that expands upon Olympus 4K monitor offerings. Backward- and forward-compatible with most Olympus processors, the new monitor provides a breadth of customizable uses at an attractive price point.
“With this new OEV321UH Monitor, customers can upgrade their endoscopy suites and ORs to have larger screens that will be important to their practices for a range of cases,” said Kevin Mancini, Business Unit Vice President, Endoscopy Division, Olympus America, Inc. “Visualization improvements can translate into meaningful practice improvements that elevate the standard of care.”
The OEV321UH video monitor features includei:
- Design compatible with future systems, including 12G-SDI connections for single cable 4K routing;
- A 32-inch screen for a larger viewing area, following an observed customer trendii
- Compatibility with a range of endoscopy and surgical systems;
- Fully adjustable design to address specific viewing needs;
- CLONE OUT function, which is used to duplicate the main monitor’s HD/4K display for use on a second monitor or on a recording device, including PIP/POP (picture-in-picture/picture-out-picture) display;
- Split screen functionality and compatibility with telecollaboration tools;
- Advanced Image Multiple Enhancer (A.I.M.E.) for sharp, vivid image of structures without increasing noise: creates enhanced texture that supports more detailed observation;
- Flat display and detachable cable cover for quick and easy monitor cleaning; access window in cable cover also allows for convenient cable routing.
Since its development of the first flexible gastroscope in 1950, Olympus has continued to bring new endoscopy and surgical innovations to market, while also delivering excellent service, support and ongoing training toward workflow optimization and operational efficiency. Olympus products and services meet a range of procedural needs, which means customers have the tools they need to stay at the forefront of their fields, as they work to help patients live longer, healthier lives.
For more information about the OEV321UH, visit here.
i Data on file with Olympus (DC00675762).