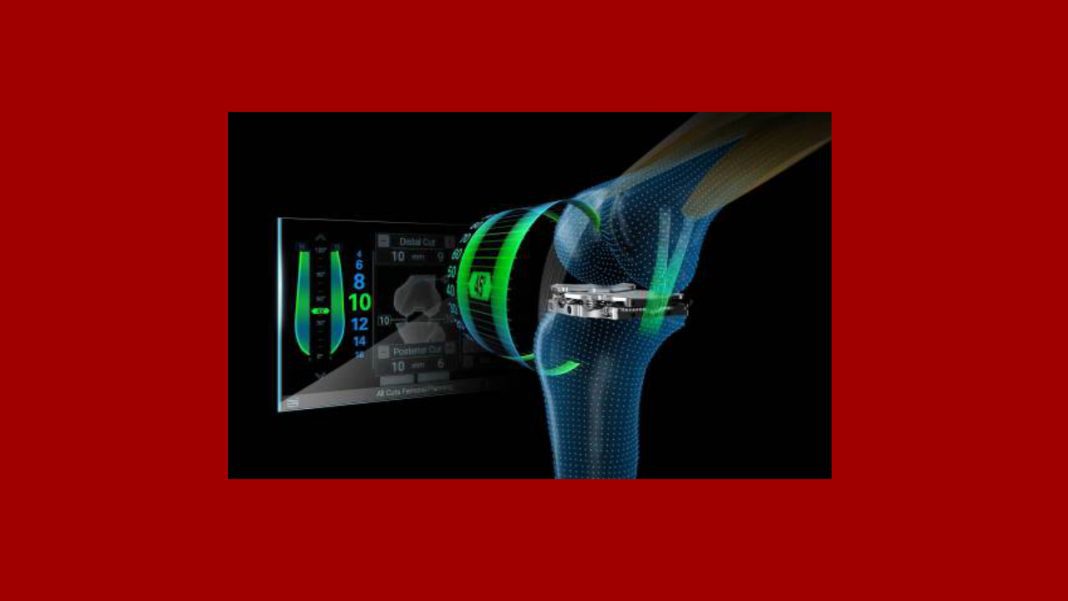
Newton™, is an innovative soft tissue management technology that combines dynamic ligament balancing and real-time guidance to bring to life a personalized intraoperative solution designed to improve total knee replacement surgery outcomes and patient satisfaction.
First Surgeries
First surgeries were performed by Hari K. Parvataneni, MD, professor of orthopaedic surgery at the University of Florida, and James Huddleston III, MD, professor of orthopaedic surgery and chief of the adult reconstruction service at Stanford University Medical Center. According to Huddleston, “Soft tissue management is an area of opportunity for progression in orthopaedics, and Exactech is leading the way. Newton is a sophisticated technology that is designed to provide real-time, patient-specific insights to help easily optimize the soft tissue with the goal of achieving the best possible outcomes for total knee patients.”
What is Newston?
Newton is an innovative intra-articular device that engages the patient’s soft tissue to help surgeons execute a personalized, well-balanced total knee replacement. By capturing active balance data through the entire arc of motion with the patella in place, Newton seamlessly integrates with ExactechGPS’s connected platform to allow surgeons to visualize a predictive plan through operative insights, without the need for external soft tissue tensioners.
Remarks from Exactech Marketing
“We are excited to introduce this game-changing technology for total knee replacement surgery,” said Adam Hayden, Exactech’s chief marketing officer and senior vice president of Large Joints. “Newton is yet another example of Exactech pioneering innovations that provide orthopaedic surgeons with intelligent insights to make smart decisions throughout the journey of care.”
Newton is the company’s latest release within its Active Intelligence platform of smart solutions that help surgeons engage with patients and peers, solve challenges with predictive tools and optimize performance. The technology is in pilot launch in the US with expanded availability planned before the end of the year.
Surgeons can experience Newton and ExactechGPS first-hand at the AAOS Annual Meeting – Booth #1035 – Sept. 1 – 3, 2021, through live demonstrations.