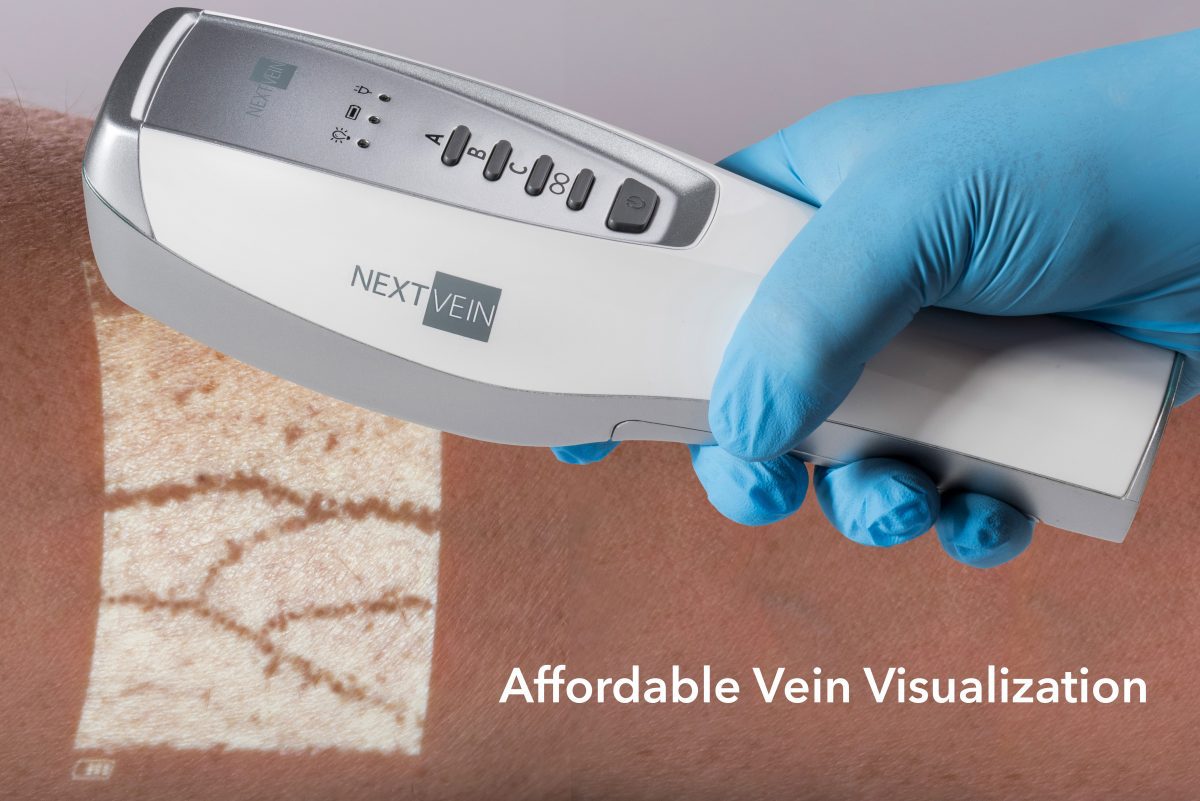
NextVein LLC company today announced a breakthrough product that makes vein visualization affordable for every clinician. The NextVein system is designed from the ground up to be a simple to use, cost-effective solution for successful venous access and vein avoidance.
This augmented reality system uses advanced camera and multicolor projection technology to project the vein pattern on the patient’s skin, providing the clinician a roadmap to the patient’s unique vasculature. The LED projection system overcomes clinician and patient concerns about lasers.
Studies have shown that vein visualization improves important venous access metrics, including:
- Improved first-time stick success rate,
- Reduction in patient pain,
- Fewer escalations,
- Cost reduction.
NextVein is the first meaningful alternative to expensive vein visualization systems. It offers the core features expected from hand-held vein visualization and adds advanced features like multicolor projection. As a hand-held device, the clinician can quickly assess the patient to identify veins. It converts to hands-free with the NextVein wheeled stand, freeing both hands for the procedure. This state-of-the-art stand provides a reliable, long-reach solution for procedures and doubles as storage while re-charging.
Vein visualization is used:
- To start IVs by nurses in hospitals and outpatient settings
- To draw blood by phlebotomists
- By Dentists, Dental Surgeons, and Dental Anesthesiologists for IV sedation
- For IV contrast in Imaging Centers
- For Aesthetic Injectables to avoid veins and visible bruising
NextVein is partnering with Medtech/Medcare (MTMC), the recognized leader in national sales solutions. Jack Moran, MTMC’s managing partner, said, “Our 125 sales executives are trusted partners to providers across the healthcare spectrum. We’re excited to bring this new high-performance, hand-held vein visualization system to our customers. Excellent product at an affordable price is the right combination and will enable our customers to deliver a higher level of care and achieve a higher level of success.”