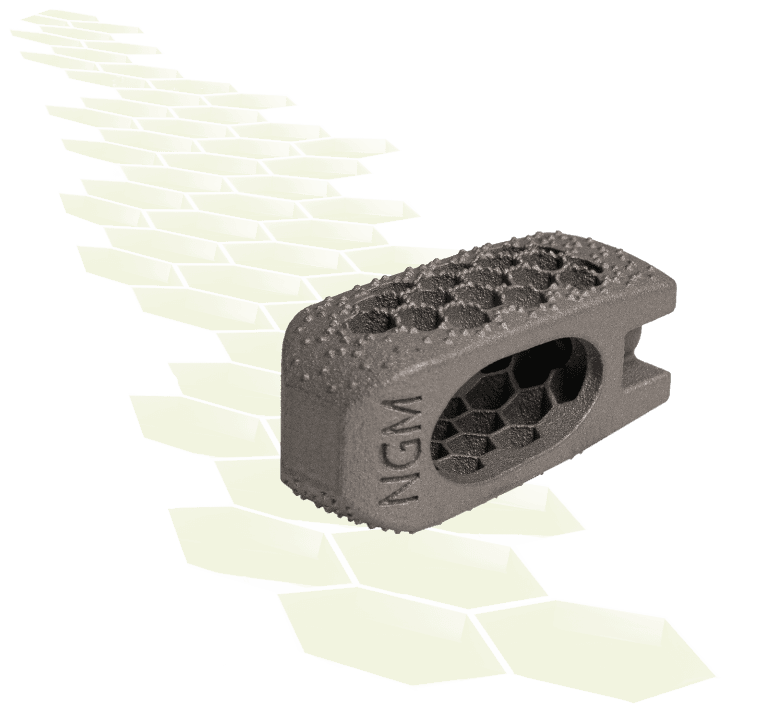
NGMedical, a German medical device manufacturer exclusively focused on creating innovative technologies for spinal applications announces the first USA implantation of its purely additively manufactured titanium BEE® PLIF.
The case was performed on February 18th, 2022 by Jonathan A. Tuttle, MD at University Hospital in Augusta, GA.
“The BEE® PLIF implant system represents a refinement of details that matter. The instruments are elegant and intuitive, and the implant is refined in structure and anatomical design. I’m pleased to incorporate BEE® PLIF into my armamentarium of surgical solutions for my patients,” said Dr. Tuttle.
Mitch White, President of NGMedical, Inc. added, “I’m excited and honored Dr. Tuttle was the first US surgeon to implant BEE® PLIF, but I’m not surprised. Dr. Tuttle had a discerning appreciation for the BEE® PLIF’s evolutionary design, and was anxious for BEE® as a surgical option. This promises to be a year of rapid growth as we introduce the inimitable BEE® platform of interbody implants across the country.”
“This successful market launch in the USA is an important step for NGMedical. The first operation with the BEE® PLIF cage in this important market is a great milestone in our international commercialization. We look forward to expanding our portfolio and footprint in the USA.” says Peter Weiland, CEO of NGMedical.
NG Medical notes the BEE® PLIF cage is another significant innovation from the team who invented the first line of additively manufactured interbody devices. The purposefully designed honeycomb endplate design reduces the risk of subsidence, while allowing fusion. The honeycomb structure allows for bony ingrowth while offering a very large graft space. Smooth lateral surfaces facilitate insert and rotate technique. BEE® PLIF is offered in a wide range of sizes up to 18° lordosis.