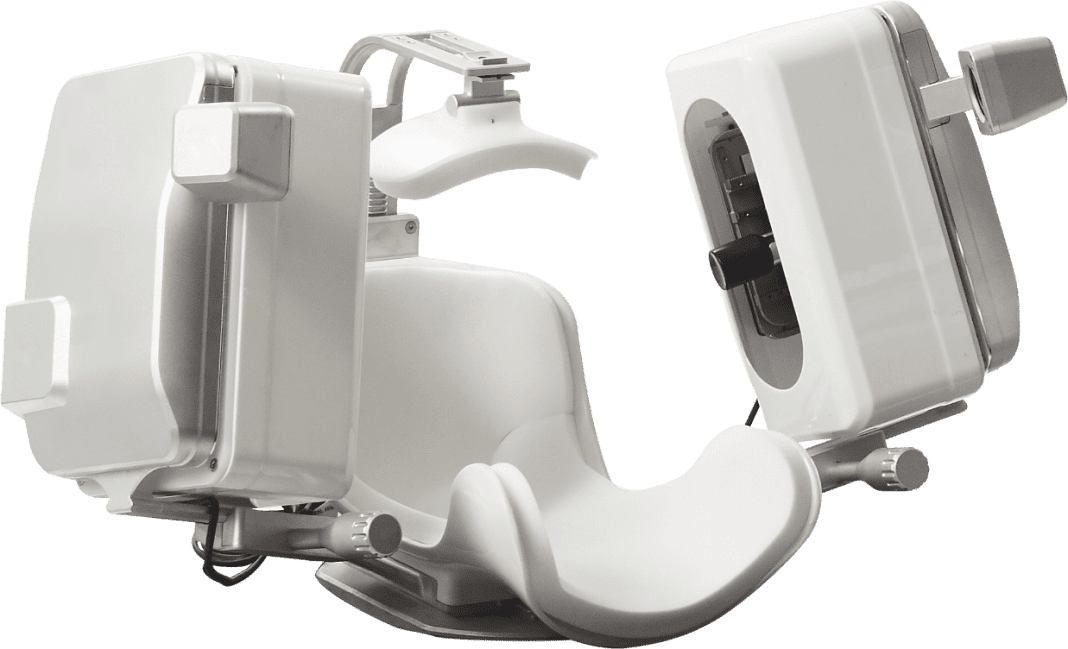
NovaSignal Corp. (formerly Neural Analytics) announced today that data published August 6th in the American Journal of Respiratory and Critical Care Medicine utilized the company’s non-invasive Lucid Robotic System to remotely assess and monitor patients with COVID-19.
Critical care teams at Mount Sinai Health System used the company’s autonomous robotic ultrasound to monitor cerebral blood flow of 18 mechanically ventilated patients with COVID-19 and revealed insights into the pathophysiology of the virus. The Lucid Robotic System integrates cerebral ultrasound, robotics, and artificial intelligence and provides physicians with cerebral blood flow data which has proven to be critical for clinical decision making and improved patient outcomes.
While monitoring these critically ill patients with the Lucid™ Robotic System, physicians discovered an overabundance of pulmonary shunting, a physical dysfunction whereby deoxygenated blood bypasses the normal circulatory system resulting in lower levels of oxygen in the blood and contributing to respiratory distress in patients with COVID-19.
Through the monitoring of blood flow through the brain, researchers found that 83% of patients had detectable microbubbles signaling vascular shunting. Additionally, the study reports that the quantity of bubbles corresponds to the level of hypoxemia and lung function in patients with COVID-19. The shunting is likely due to pulmonary dilatation (widening of the blood vessels in the lungs) leading to hypoxemia (low levels of oxygen in the blood). This discovery has advanced the understanding of the underlying pathophysiology of COVID-19 pneumonia.
“The clinical teams are challenged in the treatment of patients with COVID-19 given the need to limit their exposure. The autonomous nature of our system has enabled them to monitor patients while maintaining strict infectious disease protocols,” said Diane Bryant, Chairman, and CEO of NovaSignal. “It’s humbling and gratifying to support the healthcare providers who are so deeply committed to the care of their patients. Our company’s goal is to contribute to a better understanding of the virus, have a positive impact on patients with COVID-19, and reduce the impact of the disease.”
“The Lucid™ Robotic System was an essential component in uncovering important and unexpected findings about COVID-19 pneumonia,” added Dr. Robert Hamilton, Co-Founder and Chief Scientific Officer, NovaSignal. “We believe easy access to cerebral hemodynamics data will transform healthcare and we look forward to continued engagement with Mount Sinai as they advance the clinical understanding of the impact of COVID-19.”
Technology Drives Data Collection
Combining ultrasound with robotics and artificial intelligence enables automated, consistent, and extended monitoring of cerebral blood flow. When assessing patients with COVID-19, the Lucid™ Robotic System supports frontline worker safety by minimizing exposure to infected patients and enables physicians and sonographers to monitor patients without being in direct contact.
The Lucid™ Robotic System has been in use by leading institutions since its launch for the monitoring of blood flow in the brain in neuro-critical care units, during cardiac surgery, and in the identification of right to left shunts for patent foramen ovale (PFO – a hole in the heart and a leading cause of strokes in adults).
Study Background
The study included 18 mechanically ventilated patients with severe COVID-19 pneumonia, low lung compliance, and clinical markers of hypoxemia. Median age of the patients was 58 and 60% of the patients were men. A follow-up study is currently being conducted to generate additional findings on the pathophysiology of COVID-19.