"Initial clinical experience of the NuVision ICE Catheter supports safe and effective use with all primary and secondary safety and performance endpoints met and zero adverse events reported," commented Dr. Latib.
NuVision ICE Catheter: Positive Results From First-in-human Feasibility Study Reported At 2020 TCT Conference
Linkedin
Twitter
WhatsApp
Facebook
Copy Link
October 14, 2020
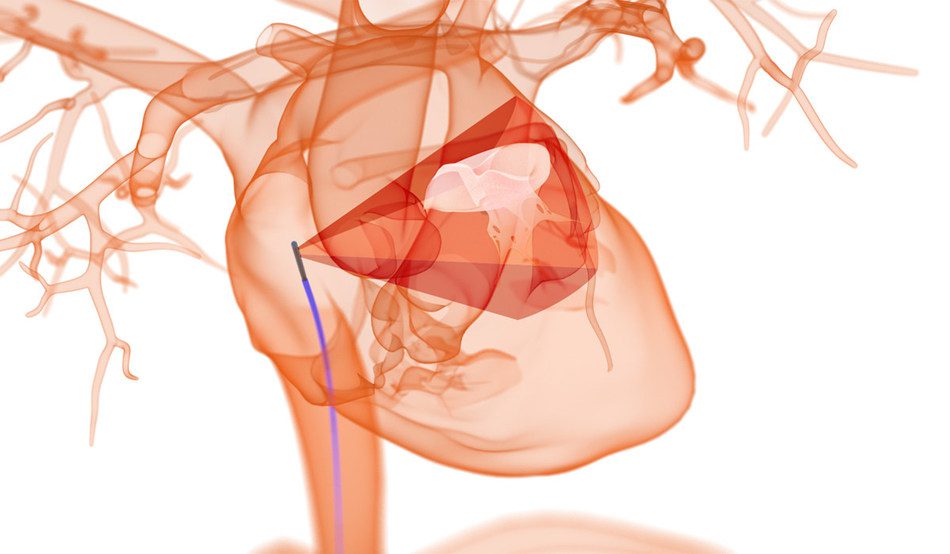
NuVision ICE Catheter: Positive results from its first-in-human (FIH) feasibility study performed by principal investigator, Dr. Adrian Ebner, Head of the Cardiovascular Department at the Universidad Nacional de Asuncion in Asuncion, Paraguay was highlighted in an abstract presentation by Dr. Azeem Latib, Director of Interventional Cardiology and Structural Heart Interventions at the Montefiore Medical Center in New York, NY as part of the 32nd Transcatheter Cardiovascular Therapeutics (TCT) meeting, the annual scientific symposium of the Cardiovascular Research Foundation. The news was announced today by Nuvera Medical.
“Initial clinical experience of the NuVision ICE Catheter supports safe and effective use with all primary and secondary safety and performance endpoints met and zero adverse events reported,” commented Dr. Latib. “This state-of-the-art 4D ultrasound technology will undoubtedly be the future of procedural guidance across a wide range of electrophysiology and structural heart procedures. I look forward to the benefit this advancement will bring to my practice and for my patients.”
One of the first virtually-supported clinical studies performed during the COVID-19 pandemic, the prospective, non-randomized, single-center FIH study investigated the feasibility of the NuVision™ ICE Catheter in visualizing anatomical structures in patients being evaluated for structural heart procedures such as atrial septal defect (ASD) or patent foremen ovale (PFO) closure, left atrial appendage (LAA) closure, and mitral or tricuspid valve repairs.
“The NuVision ICE Catheter provided me a completely new perspective in imaging of complex intracardiac structures and I could easily visualize the structures for catheter guidance,” stated Dr. Ebner. “All procedures were performed under conscious sedation and did not require intubation, which reduced overall length of stay, exposure to fluoroscopy and staffing demands. I believe NuVera’s novel solution has the potential to be an alternative to TEE for procedural guidance in structural heart interventions.”
“We are very pleased with the results of the initial patient experience and thank the outstanding clinical team for making this virtually-supported study a success,” said Todor Jeliaskov, President CEO of NuVera Medical. “We are happy to see the NuVision ICE Catheter simplified complex procedures for greater physician and patient benefit and look forward to building on this clinical experience as we prepare to bring our advanced imaging technology to market.”