Olympus, a leading global medtech company providing innovative solutions for medical and surgical procedures, announced today it will distribute EndoClot® Polysaccharide Hemostatic Spray and EndoClot® Submucosal Injection Solution, two important products developed by EndoClot Plus, Inc. (EPI), which joined with Olympus in a U.S. distribution agreement earlier in the year.
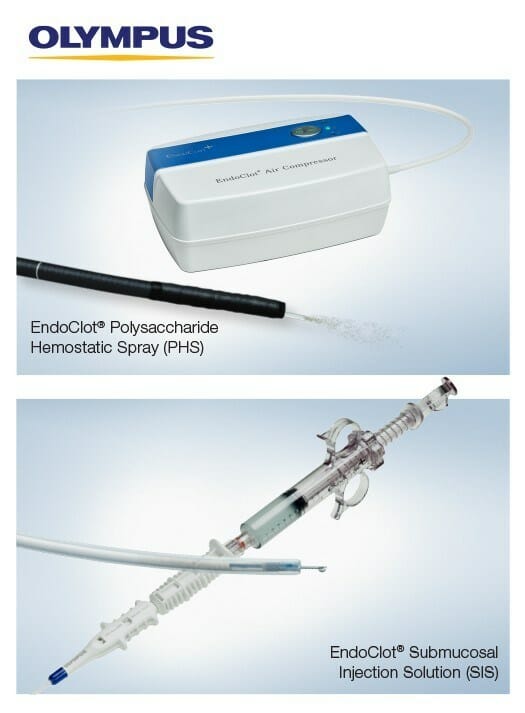
Both products are based on the EndoClot Absorbable Modified Polymer (AMP®) technology. The starch-derived AMP technology has demonstrated an excellent safety profile.i The EndoClot AMP particles work by absorbing water from blood. The dehydration process causes a high concentration of platelets, red blood cells and coagulation proteins, which helps accelerate the body’s clotting cascade.ii AMP particles are biocompatible, bioabsorbable, non-pyrogenic, starch derived and contain no animal or human components.
“Anything we can do to potentially eliminate secondary procedures with some of these more complex cases is important to our practice,” said Kenneth H. Park, MD, Assistant Professor of Medicine at Cedars-Sinai Medical Center. “We see great advantage in being able to identify the bleed and address it at the same time.”
The EndoClot PHS System enables physicians to apply an advanced powder hemostat during a procedure using controlled, consistent air pressure through a portable air compressor. Used for hemostasis of nonvariceal gastrointestinal bleeding, excluding Forrest Ia classification of bleeding, the EndoClot PHS System:
- Is indicated for use in combination with other conventional techniques, like clipping, for large and diffuse bleeds, such as those occurring in peptic ulcers, post-biopsy, polypectomy, tumor bleeding, as well as post-EMR and ESD;
- Allows for easy irrigation with water during procedures;i
- Provides control of delivery and anti-reflux capability through the applicator design, which can prevent occlusion and treat hard-to-reach bleeds;i
- Features an Air Compressor of small, portable design and provides consistent air pressure to propel powder to the bleeding site, while helping prevent the white-out effect common with CO2 propellant; and
- Helps accelerate the body’s clotting cascade: AMP particles work by absorbing water from blood, causing a high concentration of platelets, red blood cells, and coagulation proteins.
Performing hemostasis within the GI tract is a technically demanding procedure and use of EndoClot PHS and associated devices may result in patient injury including but not limited to inflammatory reaction, bowel rupture and air embolism.
The EndoClot SIS System is intended for use in gastrointestinal endoscopic procedures for submucosal lift of polyps, adenomas, early-stage cancers or other gastrointestinal mucosal lesions, prior to excision with a snare or endoscopic device. Key benefits of the EndoClot SIS solution include:i
- A long-lasting, higher lift that may create significant mucosal separation allowing for easier dissection;
- Accurate delivery to the targeted area owing to the unique spiral syringe design; and
- A lack of residual artifacts that may cause abnormalities during pathological investigations.
Use of a lifting agent during EMR/ESD/POEM and difficult polypectomy and the associated devices may result in patient injury, bleeding and/or perforation.
“Physicians are making important strides toward improved outcomes and elevating the patient experience,” said Tony Sullivan, Executive Director, Core GI Solutions, Olympus Corporation of the Americas. “Our goal is to support clinicians in treating some of the most pressing challenges across the care continuum, including achieving improved levels of hemostasis. Our work with EPI is based on these objectives, and we are excited for these launches.”
The EndoClot SIS and PHS Systems are commercially available today.