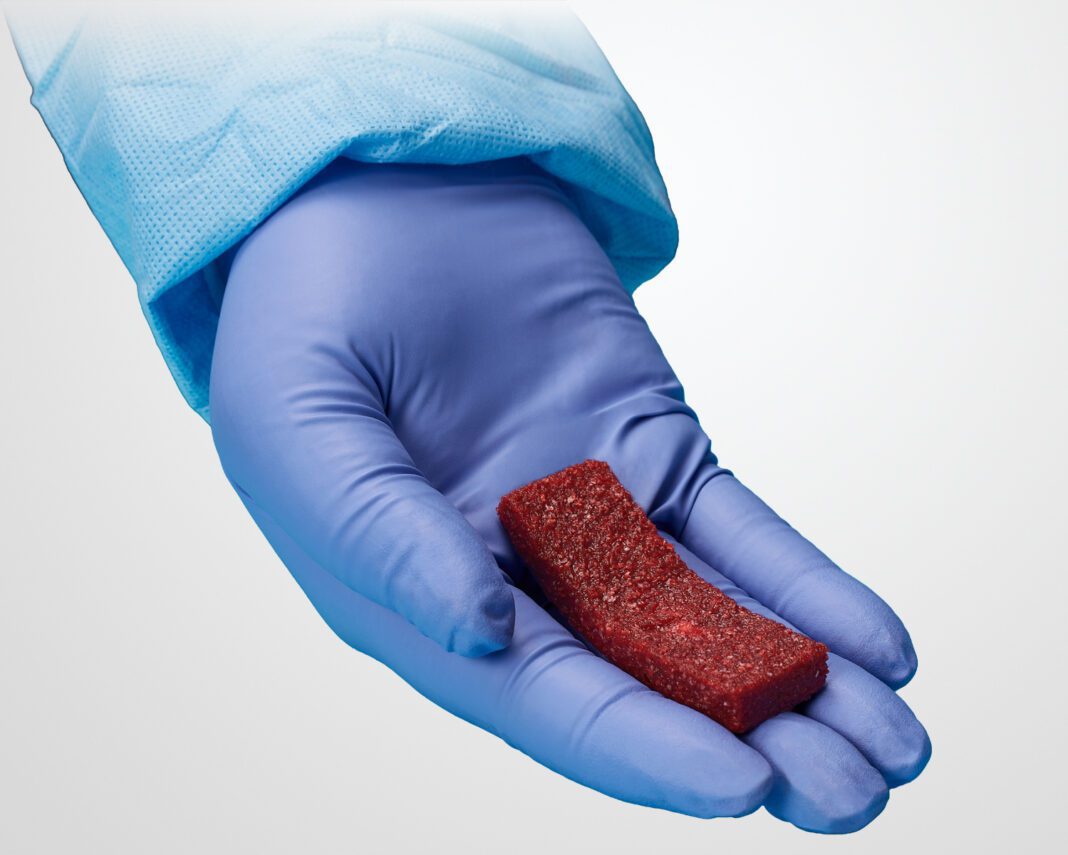
Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company with a spine and orthopedics focus, today announced the full market launch of Opus™ BA, a synthetic bioactive bone graft solution for cervical and lumbar spine fusion procedures.
Available in putty and strip formulations, Opus BA can be used to fill bone voids or gaps in the skeletal system that are not intrinsic to the stability of bony structure. Opus BA acts as a scaffold allowing bone growth across the surface that is reabsorbed and replaced with natural bone during the healing process. Easily hydrated with autogenous bone marrow or autograft with saline, Opus BA is moldable and ideal for versatile applications.
“Bioactive synthetics are a very large and growing market,” said President of Orthofix Global Spine Kevin Kenny. “We recognize that each bone substitute has its own unique advantages and surgeons have different preferences when evaluating the use of autologous, allogeneic or synthetic bone graft materials for their patients. The addition of Opus BA is another example of our commitment to providing surgeons with a wide range of solutions so they can select the best bone substitute for each patient.”
Opus BA’s osteoconductive matrix is made up of a carefully selected mix of components: carbonate apatite bone mineral, bioactive glass, and Type 1 Collagen carrier. These materials were selected for the ability to stimulate the growth, formation and resorption of bone. Opus BA undergoes a proprietary process to ensure the osteoconductive matrix has uniform distribution, mimicking the structure and porosity of cancellous bone.
Orthofix invites those attending the Congress of Neurological Surgeons Spine Summit 2022 Annual Meeting in Las Vegas, Nevada, February 24-25, 2022 to visit booth #212 to learn more about the Orthofix family of products. The latest data on Opus BA will be presented in a sponsored session entitled “The Clinical Value of Bioactive Synthetics in Spine Surgery” on February 25.
Orthofix recognizes the important role of a comprehensive offering of biologic solutions in spine and orthopedic surgical applications. The Company proudly markets and distributes the Trinity Elite™ and Trinity Evolution™ allografts with viable cells, FiberFuse™ Advanced moldable allograft, FiberFuse Strip™ preformed allograft, AlloQuent™ structural allograft, VersaShield™ amniotic membrane, Opus™ Mg Set bone void filler, Collage™ osteoconductive scaffold, and the O-Genesis™ graft delivery system. This broad portfolio gives surgeons the ability to select the best option to meet their procedural and patient needs.