- Montagu and Kohlberg to Acquire Teleflex Medical OEM in a Carve-out Transaction
- VALANX Biotech establishes Board of Directors
- Saluda Medical Raises $150M USD to Fuel Growth Following ASX IPO
- Byron Jobe Recognized as One of Modern Healthcare’s 100 Most Influential People in Healthcare
- RestorixHealth Names John Henry Senior Vice President, Growth
- Morris & Dickson LLC Signs Letter of Intent to Acquire Prodigy Health
- Understanding ACoP L8 Guidance: Your Framework for Legionella Control
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
Which are the five best hospital equipment manufacturing enterprises in China that have obtained ISO13485?
Discover the leading hospital equipment manufacturing enterprises in China recognized for their ISO13485 certification and quality standards.
Monteris Medical and Symphony Robotics Announce Strategic Collaboration to Launch AI-Driven Micro-Robotic Platform for Laser Ablation in the Brain
Alliance aims to deliver breakthrough precision with laser ablation for
complex brain tumors and expand minimally invasive care
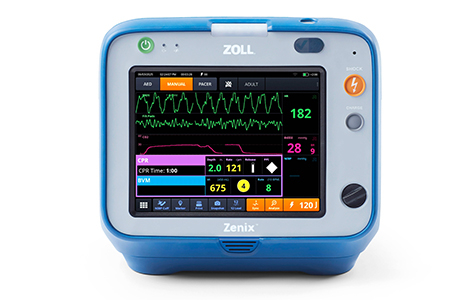
Asahi Kasei Elevates Critical Care Solutions with FDA Approval of ZOLL’s Zenix Monitor/Defibrillator
The Zenix monitor/defibrillator is designed for use in EMS and hospital settings
New AlarmSense by Nihon Kohden Aims to Alleviate Alarm Fatigue
AlarmSense™ is an advanced, data-driven analytics solution designed to intelligently streamline hospital response management and combat widespread alarm fatigue
Clinical Trials
Phase 3 Study of MR-139 for Blepharitis Update Provided by Viatris
The MR-139 3001 Phase 3 trial consisted of a randomized, placebo-controlled, double-masked prospective study, with a total of 477 patients who were randomized to receive either MR-139 or placebo, self-administered to the eyelids twice daily, treated and observed over 12 weeks.
New Study Finds Wearable ECG Monitor Detects Missed AFib in Cardiac Surgery Patients After Discharge
Continuous cardiac monitoring with Vivalink devices following surgical discharge may improve early detection of postoperative arrhythmias, according to researchers at Brigham and Women’s Hospital

Tinnitus Research: Novel Compound AC102 Makes Constant Ear Noise Disappear in Preclinical Model
These findings were recently published in the prestigious International Journal of Molecular Sciences in a joint study conducted by Erlangen University Hospital and Berlin-based drug developer AudioCure.
Biotechnology News
Biogen to Highlight Scientific Progress Across Alzheimer’s Disease at the Alzheimer’s Association International Conference 2025
Lecanemab presentations to include long-term Clarity AD data, real-world treatment insights, and a subcutaneous formulation for continued care.
Presentations on tau to explore its biological role, the development of targeted therapies and biomarkers, and the future integration of these innovations into clinical practice.
Nanochon Announces Health Canada Approval for First in Human Investigation
The trial will be led by Principal Investigator, Dr. Fathi Abuzgaya, who will collaborate with Sports Medicine Specialists, Drs. Joel Lobo, Kajeandra Ravichandiran and Marcin Kowalczuk as Sub-Investigators, at the Durham Bone & Joint Specialists (DBJS), located in Ontario, Canada.
Yourgene Health Launches LightBench Discover
Three-in-one system integrates large fragment analytics, DNA size selection and fluorometric quantification for long-read sequencing.
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

How AI Can Boldly Scale Cybersecurity Beyond Human Limits | By John Spencer-Taylor
Organizations must adopt a proactive defense strategy to stay ahead, which isn’t always easy to do with human capabilities that are limited. Fortunately, artificial intelligence can act as an auxiliary defense force and play a key role in helping improve cybersecurity efforts in a number of ways, responding faster than human teams alone.

A Wave of Drug-Free Alzheimer’s Treatment Innovation Offers New Hope | By Chuck Papageorgiou, CEO, NeuroEM Therapeutics
Chuck Papageorgiou writes: “With no effective prevention or long-term treatment, receiving a diagnosis of Alzheimer’s disease can seem like a fate worse than death for patients and their families and caregivers. However, a wave of innovation is bringing new hope to the 7 million Americans already living with Alzheimer’s disease and the 500,000 who will receive this devastating diagnosis each year.”

How AI is Transforming Vision Care | By Matthias Hofmann, Co-founder & CEO of Eyebot
“The way we think about healthcare is evolving rapidly. From AI-assisted diagnostics to telehealth expansion, technology is breaking down barriers and reshaping how people access essential medical services. In eyecare, artificial intelligence is increasingly redefining accessibility, affordability, and accuracy in vision care,” writes Matthias Hofmann.
Executives on the Move

Former Pfizer Chief Scientific Officer Dr. Mikael Dolsten, Joins MC Sciences as Independent Chairman
In this role, Dr. Mikael Dolsten will help advance the company’s mission of developing transformative and superior therapeutics targeting and controlling mast cells across multiple high-need disease indications, and progressing to clinics and patients
Advarra Appoints Brian Hart as New Chief Technology Officer
Brian Hart will drive Advarra’s technology and product strategy, bringing continuous innovation to the development and enhancement of Advarra’s solutions.
Micro Medical Solutions Appoints George Quinoy as President & CEO to Accelerate Commercialization and Drive Value Creation
Founder Greg Sullivan will transition into the role of Chief Technology Officer to focus on advancing MMS’s technology platform, next-generation product development, and clinical evidence initiatives.
FDA Medical Device Updates
MSKai Spine Imaging Software Receives FDA 510(k) Clearance for Clinical Use
Advanced software tool supports consistent, objective evaluation of lumbar spine MRI scans.
GC Biopharma Receives FDA Approval for its U.S. Plasma Collection Center in Calexico, California
With this approval, ABO Holdings is now operating all six plasma collection centers—two each in California, Utah, and New Jersey.
Carr Tech Corp Celebrates FDA 510(k) Class II Approval of FROG All-in-One Filter Needle
This transformative device advances safety and efficiency in drug preparation, underscoring the startup’s journey from solo founder to FDA-cleared innovator.
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding

Backed by $2 Million, Droplet IV is Set to Launch its Revolutionary Automatic IV-Line Flushing Device in EU & US in 2026
Droplet IV today announced the successful completion of a $2 million funding round to enable launch of its first automatic IV-line flushing device in the
Bioness Medical Announces Acquisition of the Assets and Business of Harmonic Bionics
Bioness Medical further expands pipeline of next-generation robotics offerings for Neuro Rehab.
Materna Medical Announces Ellora™ and Additional Close on $20M Series B2
The round welcomed new funds GLIN Impact Capital, Wealthing VC Club, and Citrine Angels, and was led by InnovaHealth Partners and other key existing investors.
Glytec Secures $36 Million Growth Investment to Accelerate AI-Powered Diabetes Technology Platform Innovation
This significant funding will fuel the continued expansion of Glytec’s platform, including its flagship Glucommander® SaaS solution, accelerate the development of innovative new technologies to address the growing global health crisis of diabetes and related co-morbidities, and support the continued rapid response to upcoming CMS quality measures that will hold health systems accountable for inpatient glycemic control.
Market Reports
Advancements In Imaging
NeuralCure AI Announces Partnership with Life Imaging FLA
This partnership immediately positions NeuralCure AI for rapid, real-world application, further solidifying its role as a pioneer in AI-powered healthcare solutions. Unlike traditional AI models that provide generalized insights, NeuralCure AI creates patient-specific digital twins and runs constant treatment simulations, helping physicians make more informed, data-driven decisions without replacing clinical judgment.

Philips and Mass General Brigham: Advancing Patient Care
Discover how Philips and Mass General Brigham are transforming healthcare with innovative AI solutions for better patient outcomes.

KA Imaging’s Premium Dual-Energy Mobile System Now Available For Sale In Europe Reveal Mobi Pro Has Received the CE Mark
The Reveal Mobi Pro ™ integrates KA Imaging’s Reveal™ 35C detector with SpectralDR® technology into a complete mobile X-ray solution.
Hospitals In the News

Marcus Neuroscience Institute Performs First Next-Generation Augmented Reality Spinal Surgery at Bethesda Hospital West
Marcus Neuroscience Institute, part of Baptist Health, at Bethesda Hospital West, is the first hospital in the state of Florida to perform and offer next-generation augmented reality spinal surgery with novel, advanced, high-resolution AR technology. The surgery was done by neurosurgeon Timothy O’Connor, M.D., director of minimally invasive and robotic spine surgery. Marcus Neuroscience Institute is one of the first medical centers in the world to use this next-generation augmented reality technology.

Drug Prevents Heart Failure After Heart Attacks in Mice, Study Finds
This discovery could lead to new treatments to prevent heart failure, a serious cardiac condition that develops in up to 30% of heart attack survivors within one year.
Smart Hospitals: Redefining Global Healthcare with Digital Innovation
Smart hospitals are at the forefront of a global transformation in healthcare, integrating advanced technologies to improve patient outcomes, enhance operational efficiency, and reduce costs.
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications

The Business of Bringing Medical Devices to Market
In the competitive field of medical technology, the business of launching a new device is multifaceted

What Are ELISAs? 3 Things to Know
If you’re delving into laboratory sciences, understanding the core principles and applications of ELISAs can significantly enhance your knowledge

What Are the Top Online Psychiatric Services for Professionals?
Professionals can find help that fits their needs, making mental healthcare easier to access than ever before.
Healthcare Careers
How Public Health Degrees Can Help Drive Health Initiatives
Do you care about the health and well-being of your community? Do you ever wonder how public health professionals help drive health initiatives? If so,

The Quickest Way To Get A Clinical Nutrition Degree
Health is not a new topic, but interest in it has grown, both within the public and the professional realm. Today, more people than ever

Top 4 In-Demand Careers in Social and Health Care Services to Consider
The social and healthcare services sector has been growing rapidly in recent years, and it’s showing no signs of slowing down anytime soon. With an
Nurses Corner
The Future of Nursing with AI: Studies from Columbia Nursing on Using AI for Nursing Education, Patient Care, Alzheimer’s Detection, and Mental Health
Columbia University School of Nursing is advancing nursing education, research, and practice to advance health for all. As one of the top nursing schools in the country, we offer direct-entry master’s degrees, advanced nursing, and doctoral programs with the goal of shaping and setting standards for nursing everywhere. And, as a top recipient of NIH research funding, we address health disparities for under-resourced populations and advance equitable health policy and delivery.
Press Ganey Streamlines Magnet® Readiness with AI
“Nurses play a crucial role in healthcare, and supporting their vital contributions is our highest priority,” said Jeff Doucette, DNP, RN, NEA-BC, FACHE, FAAN, Chief Nursing Officer at Press Ganey. “Nursing Intelligence speeds up and simplifies the reporting and benchmarking processes, providing nurse leaders with the insights needed for informed decision-making and enhanced care delivery.”

Hims & Hers to Offer Access to Compounded GLP-1 Injections for as Low as $99/Month to U.S. Military, Veterans, Teachers, Nurses and First Responders
Compounded GLP-1 injections are fulfilled and shipped from Hims & Hers’ affiliated pharmacies. The treatments customers receive from Hims & Hers will always come from a state-licensed, FDA-regulated facility. The company maintains high standards of safety and quality in their ingredients and transparency in operations.