- Duncan Regional Hospital Becomes First in U.S. to Implement BD Alaris™ EMR Infusion Interoperability with MEDITECH
- Salt AI Expands Leadership Bench with Biotech and AI Industry Veterans to Drive Life Sciences Growth Following $10M Funding
- Blue Cell Therapeutics appoints leading cell therapy executive Miguel Forte MD, PhD, to its Board of Directors
- HAPPE Spine Reaches 1,000 Patients with the INTEGRATE®-C Interbody Fusion System
- Sectra Reports: Large health system in southeast US adopts Sectra’s cloud service across 11 sites to ensure reliability in their delivery of excellent patient care
- ViTAA Medical Secures FDA 510(k) Clearance for AiORTA™ Plan — Marking the First Step in Its Hyper-Precise Aortic Care Platform
- Switzerland Emerges as a Safe Haven for Life Sciences
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
NICE Announces Fast Track Treatment Recommendation for Topical Oxygen Therapy
The updated NICE guidelines include topical oxygen therapy as an adjunct to standard care for diabetic foot ulcers that are not responding to treatment, reflecting increasing recognition from national health authorities of the clinical value and potential of TWO2® therapy to improve outcomes in difficult-to-heal wounds
Americans to Gain New Access to Real-Time Prescription Drug Price Information
Trump Administration announces forthcoming changes to ease approval of physician-approved treatments and inform patients up-front of the most appropriate and lowest cost prescription drugs

ClearFlow Selected to Exhibit PleuraFlow at Vizient Innovative Technology Exchange
Vizient, the nation’s largest provider-driven healthcare performance improvement company, will hold the Exchange Sept. 17 in Las Vegas
Clinical Research in Western Europe: How Belgium and France Are Setting New Standards
As clinical research becomes increasingly globalized, Belgium and France are expected to play an even greater role. Their complementary strengths make them attractive not just for European sponsors but for companies worldwide seeking reliable partners.
Clinical Trials
FastWave Medical Announces Successful First-in-Human Procedures in Study of Sola, Its Next-Generation Coronary Laser IVL System
Initial FIH procedures launch FastWave’s multi-center feasibility study evaluating the safety and performance of its laser-based coronary IVL platform.
GC Biopharma’s Phase 3 Clinical Trial Results for Hunterase Published in SCIE-Indexed Journal
Conducted at Samsung Medical Center, the Phase 3 clinical trial enrolled 24 newly diagnosed Hunter Syndrome patients with no prior treatment. It evaluated the efficacy and safety of Hunterase over a one-year treatment period.
Bionical Emas and Pharma Resources Announce Exclusive Global Clinical Trial Supply Partnership
Strategic agreement secures access to essential oncology injectables for clinical trials as European shortages intensify.
Biotechnology News
Pimicotinib Demonstrates Best-in-Class Potential with Significant Efficacy and Clinically Meaningful Improvements in Patients with Tenosynovial Giant Cell Tumor
Merck KGaA, Darmstadt, Germany, today announced the presentation of detailed positive results from Part 1 of the global Phase 3 MANEUVER trial evaluating pimicotinib in TGCT.
New Drug Approval for In-House Developed Anti-Insomnia Drug DAYVIGO (Lemborexant) in China
Eisai plans to launch this medicine in China in the second quarter of fiscal year 2025.
Caliway Biopharmaceuticals Announces Successful EOP2 Meeting with the FDA for CBL-514 in Reduction of Abdominal Subcutaneous Fat
Caliway has completed the End-of-Phase 2 (EOP2) meeting with the U.S. FDA for CBL-514, the world’s first investigational drug for large-area subcutaneous fat reduction developed under the 505(b)(1) regulatory pathway.
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

The Role of Endovascular Specialists in the Multidisciplinary Approach to Postpartum Hemorrhage | By Dr. Adam Power, Co-Founder and CMO, Front Line Medical Technologies
Postpartum hemorrhage (PPH) continues to challenge obstetric care despite ongoing medical advancements. With blood loss exceeding 500 ml in 6.3% to 13% of births and over 1000 ml in 1.9% to 2.8% of cases, PPH remains a significant concern. These figures are particularly worrying given that 500 ml is the established threshold for PPH following vaginal delivery. Cesarean sections often involve even more significant blood loss, frequently exceeding 1000 ml.

The Future of Precision Medicine Delivering ROI for the Healthcare Industry | By: Gary Turner, Managing Director, Additive Manufacturing, Ricoh USA, Inc.
Gary Turner asks: What do orthopedic surgery and additive manufacturing have in common? 3D surgical guides. This experts shares his views.

Transforming Diabetes Care: Why CGM Hardware Must Evolve for the Type 2 Population | By Dr. Chris Dawson, Head of Biosensing at TTP
The landscape of diabetes management is transforming, as continuous glucose monitoring (CGM) devices expand beyond Type 1 diabetes and into a rapidly growing Type 2 market. Changes to regulatory approvals and insurance coverage driven by improved patient outcomes have opened the door for non-insulin-dependent Type 2 patients to access CGM. Device manufacturers now face a new frontier: designing CGM hardware that meets the unique needs of an increasingly diverse patient population. For CGMs to realize their full potential in Type 2 diabetes management, it’s critical to rethink hardware design to cater to this population’s distinct requirements, improving usability, flexibility, and the quality of care.
Executives on the Move
STAAR Surgical Announces Appointment of Deborah Andrews as CFO | Forms Capital Stewardship Committee of the Board of Directors
“Deborah Andrews has blended seamlessly with the leadership team, and we quickly realized that her deep knowledge of STAAR and her skills, abilities, and approach made her the perfect choice to be STAAR’s next CFO,” said the Company’s CEO and Board member, Stephen Farrell.

Allient Inc Reports: Greg Kraus Appointed Director of Strategic Operations for Allient Defense Solutions
In this role, Greg Kraus will lead project and operational excellence initiatives across Allient sites contributing to defense market solutions, while also serving as General Manager of Allient’s Watertown, NY facility.

PolarSeal Appoints Chris Locke New CTO
Chris Locke brings over two decades of global experience in medical device R&D, technology strategy, and organisational transformation.
FDA Medical Device Updates
FDA Approves Genentech’s TNKase® in Acute Ischemic Stroke in Adults
TNKase is delivered as a single five-second intravenous (IV) bolus, a faster and simpler administration compared to the standard-of-care, Activase, which is administered as an IV bolus followed by a 60-minute infusion. Genentech will also be introducing a new 25 mg vial configuration in the coming months to support the approval of TNKase for AIS.
Co-Diagnostics Announces Intention to Submit Enhanced Version of COVID-19 Test to FDA for 510(k) Clearance
The decision by Co-Diagnostics to withdraw the submission was based on discussions with the FDA regarding the ability to detect a potential deterioration of one component of the test, related to shelf-life stability. Following dialogue with the FDA and exploring the various courses of action available, Co-Dx has determined that the best long-term solution would be to submit a version of the test that has been enhanced to address the matter raised in the 510(k) review process.
Alphyn Reports: FDA Clears Breakthrough Zabalafin Hydrogel for Atopic Dermatitis Treatment
“Zabalafin Hydrogel is on the cutting edge of an evolution in AD therapeutics to treat directly for the first time all aspects of the disease simultaneously. We believe it will be the compelling therapeutic choice to treat AD, offering excellent patient tolerability for worry free, long-term and continuous use, and comprehensive treatment for itch, inflammation, and managing the bacterial microbiome imbalance on the skin that exacerbates the disease and its flare-ups.”
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding
BDD Pharma Opens Brand New GMP Manufacturing Facility Following £2m In Funding
The BDD facility will provide cost-effective manufacturing and clinical testing for pharma and contract research organisations of all sizes.
Solace Raises $60M Series B | To Establish Healthcare Advocacy as New Standard of Care
This raise reflects accelerating market conviction that healthcare advocacy is a critical standard of care that drives better outcomes, lowers costs, and enhances patient experiences notes Solace.

SHL Medical Expands with $220M US Manufacturing Facility
SHL Medical is significantly increasing its production capacity to meet growing demand in North America while strengthening supply chain resilience.
Exciting Times Ahead for TRIMTECH Therapeutics with $31M Seed Funding
The investment will support further development of TRIMTECH Therapeutics growing pipeline of potent, CNS (central nervous system) penetrant therapeutics based on its aggregate-selective degrader molecules known as TRIMTACs. The pipeline is focused around the development of treatments for severe neurodegenerative and inflammatory disorders, including Alzheimer’s and Huntington’s disease.
Market Reports
Advancements In Imaging
KA Imaging Reports: Study Demonstrates Reveal 35C’s Impact in the ICU: Enhanced Imaging, Faster Diagnosis, and Potential CT Diversion
This is a summary of a study using the Reveal 35C detector in the ICU, to be presented at this year’s American Society of Emergency Radiology Meeting reports KA Imaging.
MAUI Imaging Emerges From Stealth With $4 Million Department of Defense Contract To Support Trauma Medicine
MAUI Imaging CEO and co-founder David Specht. “The feedback we have received from physicians and technologists highlights the profound need for a new ultrasound-based technology that enables imaging of all types of tissues. That need is most pronounced in trauma medicine, which is a major focus of MAUI’s collaborative development efforts. Going forward, MAUI will be able to supply the volumetric imaging data for AI tools that predominantly come from CT and MRI.”
Life Molecular Imaging and PharmaLogic announce Neuraceq® availability in Denver
“By partnering with PharmaLogic and offering Neuraceq® in Denver, Life Molecular Imaging is excited to expand access of Neuraceq® for the detection of beta-amyloid plaques in the brain. We believe this additional access benefits physicians and patients, as well as our pharma partners for routine clinical and research use. We are excited to collaborate with Pharmalogic today and look forward to more opportunities in the future. ” said Ludger Dinkelborg, Ph.D., Managing Director at LMI.
Hospitals In the News
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications

How Local SEO Helps Private Practices Compete With Big Healthcare Chains
Local SEO is no longer a “nice to have.” It’s a necessity for any private clinic that wants to grow in 2025 and beyond..

What To Look for in Virtual Medical Scribing Software
Virtual Medical Scribing Software: The healthcare industry is constantly changing, and the introduction of medical scribing software is revolutionizing how healthcare professionals operate. With the
IoT in Healthcare: 5 Revolutionary Devices
IoT in Healthcare: The healthcare industry is rapidly adopting IoT technology. According to a report by MarketsandMarkets, the global market for IoT in healthcare will grow from $127.7 billion in 2023 to $289.2 billion by 2028.
How Individual Coverage Health Reimbursement Arrangements Are Changing Health Benefits
Many employers researching innovative ways to redesign their benefits programs are now looking into ADP’s plans and similar offerings to see how they can deliver a personalized experience without sacrificing business stability.
Healthcare Careers

Looking for a Career in the Medical Sector?
The medical field is something you cannot take lightly nor is it for the faint of heart. It requires a person’s full attention, dedication, and
How Can You Improve Your Medical Career?
Are you looking for ways to advance your medical career? Growing as a professional in the medical field can be difficult and complex. It involves

What Are the Duties of a Pharmacy Technician?
The Healthcare industry is constantly growing. As the industry grows, changes and evolves, the demand for professionals in the field grows concurrently with it. Pharmacy
Nurses Corner
Archer Review Unveils Cutting-Edge Products Catering to Every Stage of the Nurse Life Cycle
“Our mission at Archer Review has always been to empower nursing students with the best tools and resources to succeed,” said Morgan Taylor, DNP, CPNP-PC, CNO of Archer Review. “With the launch of these new products, we are extending our support to cover every stage of the nurse life cycle, from aspiring students to practicing professionals.”
University of Phoenix College of Nursing Dean Dr Raelene Brooks Featured Speaker at Healthcare in the Age of Personalization Summit
“Patients deserve personalized, empowered care by their healthcare providers,” states Dean Dr Raelene Brooks. “Healthcare providers are experts in their field and when committed to the very best patient care and experience, they can be powerful and collaborative advocates working alongside patients and other providers. I look forward to sharing insights from our own experience training and empowering our nursing students to also be patient advocates.”
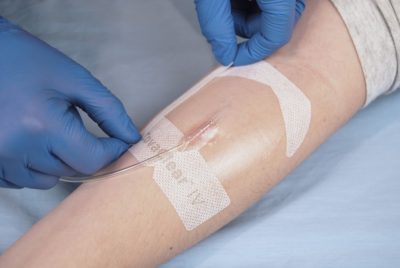
Covalon Technologies Presents Compassionate Care Technology at the Infusion Nurses Society (INS) Annual Meeting & Exhibition in Kansas City, MO
Not all vascular access dressings are made the same, and Covalon Technologies is excited to showcase more compassionate solutions at the Infusion Nurses Society Annual Meeting and Exhibition.
![Nooro Foot Massager Reviews WARNING EXPOSED!! [TOP 7 INSTRUCTIONS!] You Need to Know!!! - Medical Device News Magazine Nooro Foot Massager](https://infomeddnews.com/wp-content/uploads/2024/03/nooro-foot-massager-400x246.png)



