- Philips Advances Minimally Invasive Therapy Procedures In Prostate Cancer Care with FDA 510(k) Clearance for Image-guided Navigation Technology
- Aidoc Secures $150M for CARE, its Healthcare Foundation Model, to Transform Clinical Decision-Making for 100 Million Patients
- Micro Medical Solutions Appoints George Quinoy as President & CEO to Accelerate Commercialization and Drive Value Creation
- SurGenTec® Achieves Key Milestone with Expanded Indications for OsteoFlo® HydroFiber™
- Sleep Apnea Threatens Women’s Cognitive Health | Ignored and Undiagnosed
- Cooler Heads Secures Series A Funding to Revolutionize Scalp Cooling Technology for Chemotherapy Patients
- DualityBio Reports: Next-Generation HER3 ADC DB-1310 Granted FDA Fast Track Designation
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
IBA signs Proteus®ONE system contract with AIG in Hyderabad, India
The contract includes the supply of a Proteus®ONE system and a Quality Assurance package from IBA Dosimetry. Proteus®ONE is the market leading compact proton therapy system, upgradable over time to continue to offer the latest technology to IBA users. The system will also include DynamicARC®[2] beam delivery capabilities, once this feature has received regulatory clearance.
Surmodics Announces Successful Early Clinical Use of Pounce™ XL Thrombectomy System, Suitable for Non-Surgical Removal of Thrombi and Emboli from Iliac and Femoral Arteries
“We’re excited about the positive feedback we’ve received from early users of the Pounce XL System,” said Gary Maharaj, President and Chief Executive Officer of Surmodics. “The addition of this larger-profile device to the Pounce Thrombectomy Platform fulfills our goal of providing physicians a standalone solution for rapid removal of acute or chronic peripheral arterial clot throughout the lower extremity. With hospitals under growing pressure to reduce costs, we believe the standalone Pounce Thrombectomy Platform may help reduce the need for hospitalizations and follow-up procedures.”
IVOS Medical Announces BOSS G4™ Video Laryngoscope Sheath Designed to Improve Intubation Safety in High-Risk Patients
“Our BOSS G4 is designed to empower healthcare providers and first responders, and to raise the standard of care in intubation by improving outcomes in emergency intubation situations,” said Gabriel Punsalan, CRNA, MS, CEO and Co-Founder of IVOS Medical, Inc.
R3 Vascular Secures WCG IRB Approval and CMS Category B Medicare Coverage for the ELITE-BTK Pivotal Trial of its MAGNITUDEÒ Drug Eluting Next Generation Bioresorbable Scaffold for Below-the-Knee Peripheral Arterial Disease
R3 Vascular Inc., a medical device company dedicated to developing and providing novel, best-in-class bioresorbable scaffolds for treating peripheral arterial disease (PAD), is pleased to
Clinical Trials
Sedana Medical Completes Patient Recruitment for INSPiRE-ICU 1 Clinical Trial in the US
Peter Sackey further elaborated: “Once the 30-day follow-up of all patients is complete, we will enter into an intense phase of final monitoring, data cleaning and transfer to our statistician team for analysis. In parallel, the long-term outcomes at 3 and 6 months will be collected centrally by the Critical Illness, Brain Dysfunction, and Survivorship team at Vanderbilt Medical Center. With this parallel approach, we expect topline results in the autumn of this year and a swift regulatory submission in Q1, 2025”.

Unlocking New Hope: Alzheimer’s Patients Join Amyloid-Targeting Therapy Study
The study is designed to assess the clinical utility and workflow benefits of Swoop® system images acquired at infusion centers and clinics to help physicians detect amyloid-related imaging abnormalities (ARIA) in Alzheimer’s patients receiving amyloid-targeting therapy at the times specified in the labeling (before the fifth, seventh, and fourteenth infusions).
Indaptus Therapeutics Presents Positive Mechanism of Action Data at the American Association for Cancer Research Annual Meeting
Dr. Michael Newman, Indaptus’ Founder, Chief Scientific Officer, and lead author, commented, “The new data are consistent with our preclinical animal tumor model studies and provide evidence for our hypothesis that patented Decoy bacteria can activate a wide range of innate and adaptive human immune cells involved in fighting tumors. This aligns with what we’ve observed in our ongoing Phase 1 clinical trial of Decoy20 – broad immune activation, as evidenced by transiently increased levels of many key cytokines and chemokines following single dose administration. These findings bolster our confidence in Decoy20’s potential as a multifaceted immunotherapy.”
Biotechnology News
Addex Therapeutics to Present at the Thirteenth London International Cough Symposium (13th LICS)
Mikhail Kalinichev, Head of Translational Science, will present at the Thirteenth London International Cough Symposium taking place July 18 – 19, 2024 at Imperial College in London, U.K. reports Addex Therapeutics.
Medsenic, a Subsidiary of BioSenic SA, Extends Key Patent to the United States
Medsenic SAS, subsidiary of BioSenic SA, is exploring the therapeutic use of ATO in several indications with clinical success. The company is also making significant progress in elucidating the mechanisms by which ATO modulates immune responses and the ability of certain metal ions to enhance this therapeutic potential.
Valneva Receives Marketing Authorization in Europe for the World’s First Chikungunya Vaccine, IXCHIQ ®
IXCHIQ ® is the world’s only licensed chikungunya vaccine available to address this unmet medical need. In accordance with the International Recognition Procedure (IRP)3, Valneva has also submitted a Marketing Authorization Application (MAA) to the UK Medicines and Healthcare products Regulatory Agency (MHRA). An additional marketing authorization application is under review by the Brazilian Health Regulatory Agency (ANVISA) to make the vaccine available in certain Low- and Middle-Income Countries (LMIC), with potential approval in 2024.
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

Why 3D Printers Are Ready to Transform Medical Device Design and Delivery | By Francois Minec, Global Head of 3D Polymers, HP Personalization & 3D Printing
One of healthcare’s scarier little secrets is how extremely tricky it can be to accurately place screws into spinal pedicles during surgery. In fact, a few years ago five experienced surgeons were reportedly asked to manually insert hardware used to strengthen fused vertebrae into cadavers. When researchers reviewed a follow-up CT scan, they found placement error rates as high as 41%. Even worse, 21 of the screws surgeons implanted penetrated the medial wall of the pedicles and entered the vertebral canals.

Revolutionary Technology Will Soon Transform Cardiac Arrhythmia Care | By Steven Mickelsen, M.D.
Over the past four years, the pulsed field ablation (PFA) arms race has become very heated. Once just a concept, PFA is nearly here as companies compete to be first to market in the U.S. Read on.

Optimize Consumer Medical Device Sales with AI-Power
Incorporating AI-based tools in the sales process can increase revenue and improve customer satisfaction. These tools can transform how sales teams function, allowing them to focus on what matters most – meeting customer needs and driving sales growth.
Executives on the Move
FDA Medical Device Updates
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding
Gleamer raises €27 Million in Series B Funding Round
This new investment will enable Gleamer to expand its portfolio of solutions, strengthen its European and American teams, and accelerate its international development plans. Total funds raised to date amount to €36 million (which includes a Series A funding round of €7.5 million in 2020 and seed funding of €1.5 million in 2018).
Presidio Medical Announces $72M Equity Fundraising Led by Deerfield
In conjunction with this financing David Neustaedter, Ph.D., Venture Partner, Deerfield Management Company, has joined the Presidio Medical Board of Directors.
Vertex Ventures HC Announces $200 Million Fund III to Drive Innovation in Healthcare
Since its founding in 2014, Vertex Ventures HC $550M assets under management and invested in more than 25 portfolio companies to bring transformative therapies and healthcare technologies to patients, including nearly 20 clinical-stage assets and three products cleared by the US Food and Drug Administration
Tagworks Pharmaceuticals Announces $65 Million in Series A Financing to Advance Click-to-Release Therapeutics
The financing will support the advancement of TGW101, Tagworks’ lead click-cleavable ADC program, and the company’s proprietary Click-to-Release platform.
Market Reports
Advancements In Imaging
Ewellix Demos Set of New Products for the X-ray Suite
Ewellix noted the new products include lifting columns and sophisticated control units based on the company’s innovative SmartX digital technology.
Philips and TriHealth Announce Multi-year Partnership at zheart of New TriHealth Heart & Vascular Institute On the Campus of Bethesda North Hospital
The partnership also consists of technology management plans, helping to ensure the health system can benefit from the latest technology innovations which will help them drive their strategy and deliver on their mission notes Philips.
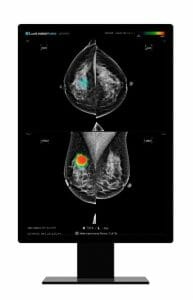
Lunit and Agfa HealthCare Bring Breast Imaging AI Solution to Dubai Academic Health Corporation Dubai Hospital
Dubai Academic Health Corporation (DAHC) Dubai Hospital to modernize its Breast Cancer Diagnostic and Screening program with Agfa HealthCare’s RUBEE for AI framework and Lunit INSIGHT MMG algorithm.
Hospitals In the News
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications

The Benefits Patients And Clients Have Receive Due To The Remarkable Rise Of Technology
The medical world is ever-evolving and it’s an amazing sight to behold. The world of healthcare has so many different sectors, and they do so

Role of Artificial Intelligence in Healthcare: Exploring AI Applications in Diagnosis, Treatment Planning, and Patient Care
In the ever-evolving landscape of healthcare, one technology stands out as a transformative force, promising to revolutionize how medical practitioners diagnose, plan treatments, and deliver

Most Common Myths and Misconceptions About Healthcare Technology
In today’s digital age, healthcare technology has emerged as a transformative force, revolutionizing patient care, medical procedures, and data management. However, as with any revolutionary

Advantages, Challenges, and the Future of Remote Healthcare Consultations
Over the past few years, remote healthcare consultations have greatly changed the healthcare industry. With the help of technology and easy internet access, medical professionals
Healthcare Careers
Nurses Corner

Skills And Qualities You Need To Be A Mental Health Nurse
Considering this career path? Read on to find out what you need to know!
AANP Applauds Signing of Law to Support Health Care Workers Suffering From Burnout
AANP is the largest professional membership organization for nurse practitioners (NPs) of all specialties. It represents the interests of the more than 325,000 licensed NPs in the U.S.

Should Nurses Improve Their Standing With A FNP Certificate
FNP Certificate This article discusses the benefits of getting an FNP certificate and how it can help improve your standing as a nurse. Read on.