Amend Surgical Announces ISO 13485 Certification, Advancing Toward Launch of Amend Tissue Tape™
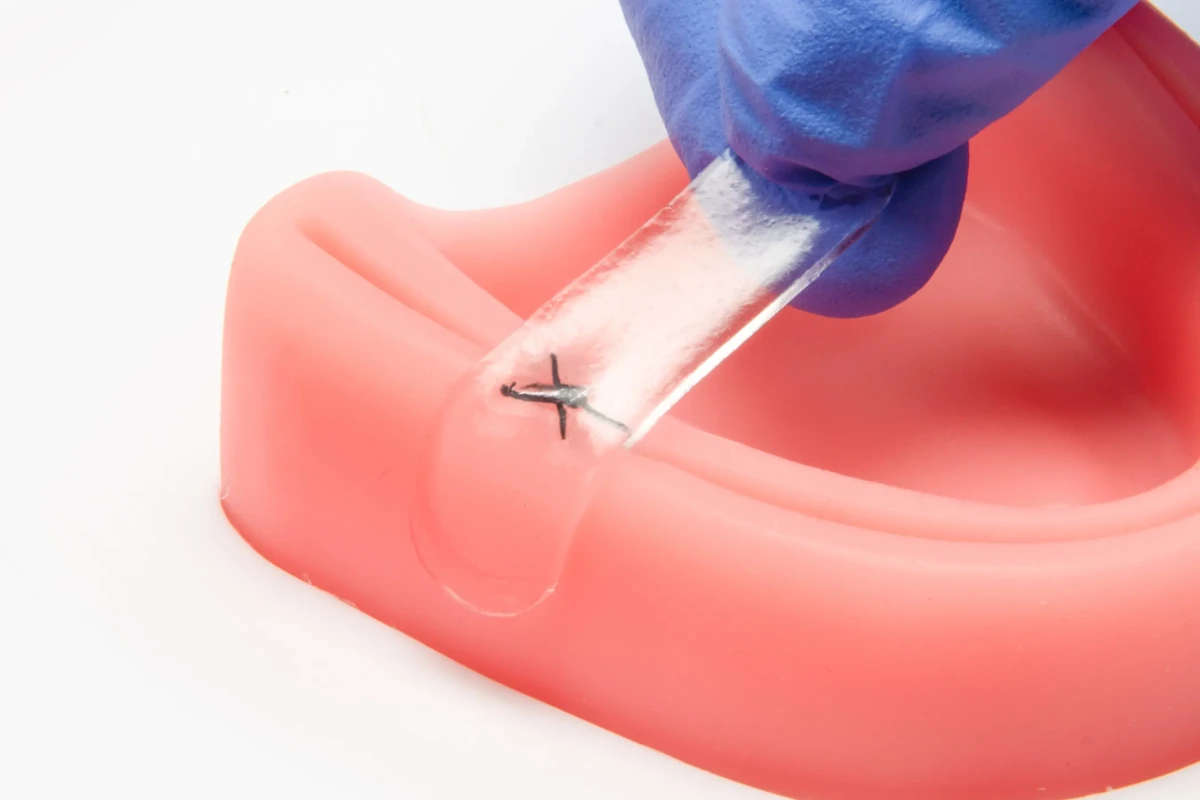
This milestone further strengthens the company’s manufacturing readiness as they advance their pipeline of next generation oral surgery solutions including the launch of Amend Tissue Tape™ and NanoFUSE™ for dental applications later this year