- Immunic Presents Key Vidofludimus Calcium Data at the ACTRIMS Forum 2025
- Tom Arnold Appointed CEO of ViCentra
- Dr. Samuel A. Joseph Jr. Named Clinical Assistant Professor of Orthopaedic Surgery at HCA/USF Orthopaedic Program
- Fosun Pharma Received NMPA Approval for Wan Ti Le | (Tenapanor Hydrochloride Tablets)
- EPO Grants Key CRISPR Patent to ERS Genomics CVC Portfolio
- Birgitte Volck Appointed Non-Executive Director at OMass Therapeutics
- DT Research Extends Comprehensive Medical Computing Line with DT514 All-in-One Computers
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook

VTT Creates a Biodegradable ECG Patch
The new nanocellulose and carbon ECG patch aims to reduce the carbon footprint of plastics and electronics for one of the world’s largest waste-producing sectors: healthcare. VTT is currently looking for potential partners to bring manufacturing to industrial scale.
Inspira™ Technologies will Reveal the New ALICE Device at the World’s Largest Extracorporeal Life Support Conference
“Revealing the ALICE™ device to the medical community at the ELSO conference in the U.S. marks an important milestone for the company and the first step towards Inspira’s market penetration. At the conference we plan to initiate commercial engagements,” commented Joe Hayon, President of Inspira Technologies.
Virtuoso Surgical Unveils Design of Groundbreaking Robotic Surgery System
“Virtuoso gives surgeons their hands back, equipping them to lift tissue, apply tension and maintain traction – in tight spaces within the body,” said S. Duke Herrell, III, MD, FACS, CEO, Co-Founder and Chief Medical Officer of Virtuoso Surgical. “These are groundbreaking maneuvers in endoscopic/endoluminal surgery that are not possible with today’s instruments.”
Iterative Health Announces Upcoming U.S. Availability of SKOUT®: Real-Time AI for Polyp Detection
SKOUT is indicated as a computer-aided detection tool to assist qualified and trained endoscopists in identifying potential colorectal polyps during colonoscopy examinations in adult patients undergoing colorectal cancer screening or surveillance.
Clinical Trials
Watkins-Conti Completes Stress Urinary Incontinence Yōni.Fit Device Trial
Seventy one (71) participants enrolled in a randomized, controlled, single-blind, multi-center study of the Yōni.Fit device in women with stress urinary incontinence.
NICO Corporation’s ENRICH Trial Evaluating Minimally Invasive Parafascicular Surgery (MIPS) for Intracerebral Hemorrhage (ICH) to be Presented at the 2023 AANS Annual Scientific Meeting During Plenary Session I
The ENRICH trial results were accepted as a late-breaker and will be presented on Saturday, April 22 during an oral plenary session beginning at 8:30 a.m. PT (11:30 a.m. ET) by co-lead ENRICH investigator, Gustavo Pradilla, M.D., associate professor of neurosurgery at Emory University School of Medicine and chief of neurosurgery at Grady Memorial Hospital.
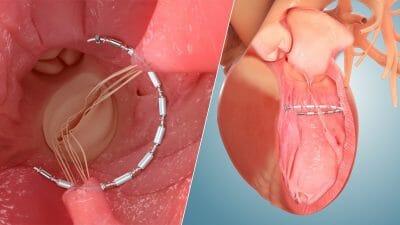
Ancora Heart and egnite, Inc. Announce Strategic Partnership to Accelerate Clinical Trial Enrollment for Heart Failure Patients
Mark Miles, chief commercial officer of Ancora Heart. “This partnership with egnite and The Christ Health Network will allow us to deploy AI tools such as egnite’s Trial Accelerator and expedite identification of eligible candidates for the CORCINCH-HF trial.”
Biotechnology News
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

Changes in Federal and State Law That Have Expanded Medical Assistants’ Scope of Service
Donald A. Balasa, JD, MBA, CEO, and House Legal Counsel, American Association of Medical Assistants writes about the significant changes in laws and standards that have empowered medical assistants to go the extra mile and band together with their fellow health professionals to respond to one of the most serious health crises in the last one hundred years.

Bernard Ross On Tackling the Financial Burden of Transplantation Surgery with Medical Technology (MedTech)
Bernard Ross, CEO of Sky Medical Technology, returns to Medical Device News Magazine to discuss how medical technology (MedTech) can help reduce the financial burden of organ transplantation costs in the US.

Jian Zhang, CEO & Founder, Noah Medical Article: Importance of Investing in Healthcare and MedTech R&D
Jian Zhang explains why investing is a crucial component of the development of tech-enabled solutions. Read what he has to say.
Executives on the Move