- Bariatric Surgery: Balancing Medications and Surgical Interventions
- SamanTree Medical Highlights Research Showing 67% Reduction in Reoperation Rates for Breast-Conserving Surgery at the 2024 SABCS
- New Data From SonarMD Finds that Anxiety Increases Risks for Inflammatory Bowel Disease Flares and Disease Severity
- Ceryx Medical Initiates First-In-Human Study of the Cysoni™ RSA Pacemaker
- How Prioritizing Employee Mental Wellness Creates a More Positive, Engaged Workforce | By Dawn Griffin, chief human resources & diversity officer at TRIMEDX
- From Standardized to Specialized: Revolutionizing Lung Cancer Care | By Ofer Sharon, MD, CEO of OncoHost
- Anocca Announces Submission of Clinical Trial Application for Multi-asset Trial VIDAR-1 in Advanced Pancreatic Cancer
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
Eyenuk Secures European Union Certification for Autonomous AI-Powered Detection of Age-Related Macular Degeneration and Glaucoma
“Achieving MDR certification is another major milestone for Eyenuk and a result of our early adaptation to the more stringent requirements of the new regulation,” said Kaushal Solanki, Chief Executive Officer and Founder of Eyenuk. “Moreover, this certification expanding our ability to market the EyeArt platform for two additional disease conditions in the EU reflects the flexibility of our AI-based platform and the depth of the clinical research conducted across the globe validating its effectiveness.”
CEYEBER Develops First-Ever Intraocular Smart Lens: The Third Eye
“We’re very excited about our revolutionary solution for age related macular degeneration (AMD), accommodative vision correction and the long-term prevention of blindness. According to recent estimates, close to 200 million people globally are affected by AMD which is expected to grow to 288 million people by 2040. CEYEBER will have a profound impact on the quality of life and vision for millions of people worldwide,” explained Robert Edward Grant, Founder and CEO of CEYEBER.
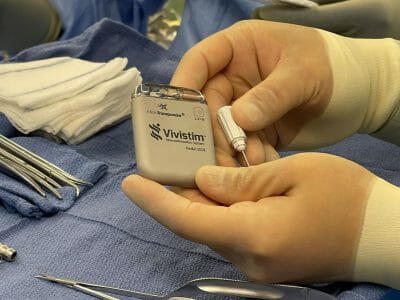
MicroTransponder Secures CMS Transitional Pass-Through Status & New Technology Add-On Payment for Its Breakthrough Stroke Rehabilitation System, Vivistim
The Vivistim Paired VNS System is an FDA Breakthrough Device that was granted pre-market authorization (PMA) approval on August 27, 2021. Unlike other neurostimulation technologies, the Vivistim System is used in conjunction with rehabilitation therapy to help ischemic stroke survivors who have chronic arm and hand deficits improve their upper limb function.
Medical iSight Establishes Neuroradiology Collaboration with NYU Langone Health
Medical iSight CEO, Justin Hall-Tipping, commented: “We are delighted to be working with NYU Langone Health and their world-class doctors to advance our groundbreaking interventional software and deliver on our vision to unite surgical digital information within a single cognitive platform, to make it universally accessible, thereby resulting in better patient outcomes.”
Clinical Trials
MedAlliance Leads US Sirolimus DEB race: First US Patient enrolled into SELUTION SLR Coronary Sirolimus DEB Study
SELUTION SLR is commercially available in Europe, Asia, the Middle East, and the Americas (outside USA) and most other countries where the CE Mark is recognized. Over 10,000 units have been used for patient treatments in routine clinical practice or as part of coronary clinical trials. Centres interested in participating in this study, please contact MedAlliance.
VEXAS Syndrome Study Offers First Glimpse of How Many Suffer From Previously Unknown Illness
In the new VEXAS syndrome study, publishing in the Journal of the American Medical Association (JAMA) online Jan. 24, researchers analyzed the electronic health records of 163,096 mostly white men and women in Pennsylvania who agreed to have their blood DNA screened for signs of genetic disease. Twelve were found to have the UBA1 mutation, with all experiencing VEXAS symptoms.
Ra Medical Systems Announces the Presentation of Clinical Data at the European Heart Rhythm Association Congress
David Jenkins, Ra Medical Systems Executive Chairman. “All four abstracts with VIVO clinical data submitted by leading physicians to the Congress were accepted for presentation following an extensive peer-review process. To have one-hundred percent acceptance of submitted abstracts is rare and I believe indicative of the positive impact VIVO is having on ventricular ablation procedures. We view this recognition as a major milestone in gaining clinical acceptance by both EP thought leaders and the larger medical community.”
Biotechnology News
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

Smart Noise Cancellation: A Groundbreaking Advance in X-ray Image Quality
By Jim Sehnert, Ph.D., Director of Data Science Research at Carestream Health. Read on:

Creating Stability at a time the Medical Industry Experiences Rising Pressures
Ofir Paldi, CEO of Shamaym lends his views and thoughts on this most important subject and how medical device company team members can keep up with the pressures. Read on.

The Role of Health-tracking Technologies in the Future of Healthcare
The next wave of technology has been accelerated greatly by the global pandemic, but what does the next decade look like in healthcare, both in facilities and in the home, writes Dr. Peter Small, Chief Medical Officer with Hyfe. Read more.
Executives on the Move





