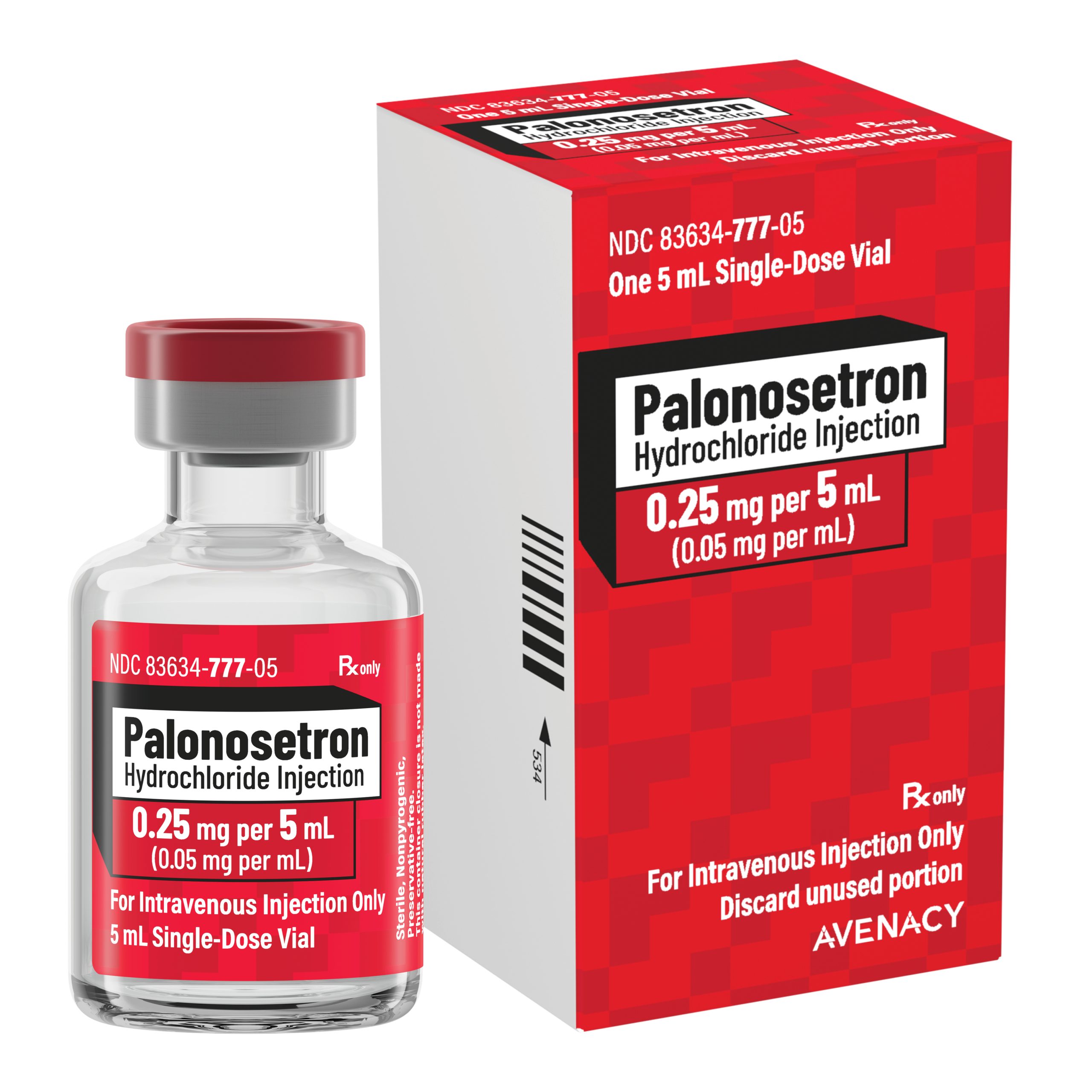
Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Palonosetron Hydrochloride Injection, USP in the United States as a therapeutic generic equivalent for Aloxi® as approved by the U.S. Food and Drug Administration. Palonosetron Hydrochloride Injection, USP is indicated as an antiemetic.
Avenacy’s Palonosetron Hydrochloride Injection, USP is available in 0.25 mg/5 mL (0.05 mg per mL) single-dose vials. In line with Avenacy’s mission to champion patient safety and streamline patient care, Palonosetron Hydrochloride Injection, USP will feature the Company’s highly differentiated packaging and labeling to support accurate medication selection.
Avenacy will begin shipping Palonosetron Hydrochloride Injection, USP to wholesale partners this week. The Company is supported by a global network of development and contract manufacturing partners that have undergone successful FDA inspections based on cGMP-standards.
Palonosetron Hydrochloride Injection, USP had U.S. sales of approximately $20 million for the twelve months ending in June 2023.1
Approved Indications:
For Adult Use
Palonosetron hydrochloride injection is indicated in adults for prevention of:
- Acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (MEC),
- Acute nausea and vomiting associated with initial and repeat courses highly emetogenic cancer chemotherapy (HEC),
- Postoperative nausea and vomiting (PONV) for up to 24 hours following surgery. Efficacy beyond 24 hours has not been demonstrated.
As with other antiemetics, routine prophylaxis is not recommended in patients in whom there is little expectation that nausea and/or vomiting will occur postoperatively. In patients where nausea and vomiting must be avoided during the postoperative period, palonosetron hydrochloride injection is recommended even where the incidence of postoperative nausea and/or vomiting is low.
For Pediatric Use
Palonosetron hydrochloride injection is indicated in pediatric patients 1 month to less than 17 years of age for prevention of:
- Acute nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy, including highly emetogenic cancer chemotherapy.
Please see link for Full Prescribing Information including the Boxed Warning.
Aloxi® is a registered trademark of Eisai Inc.
1Source: IQVIA