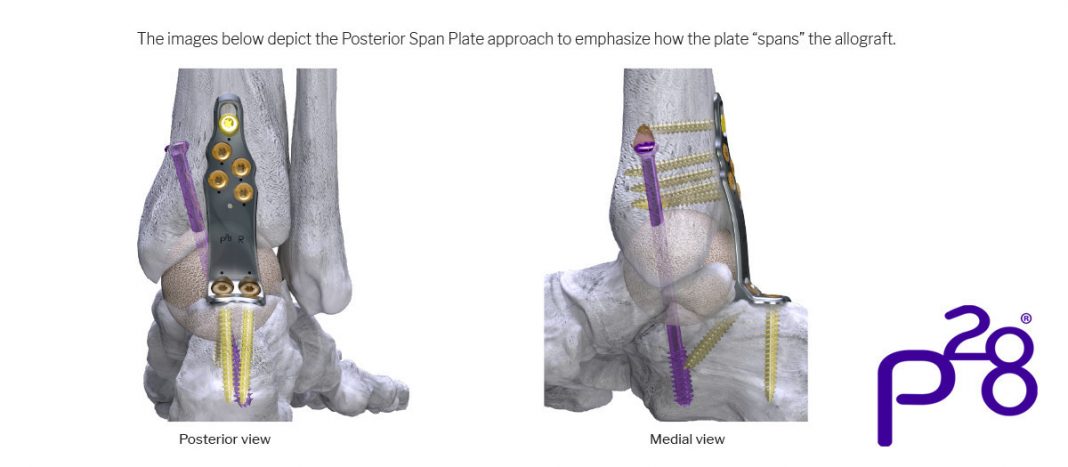
Paragon 28® is pleased to announce the launch of its Silverback™ Span Plating System, which was specifically designed to be used in combination with a structural allograft or a titanium cage in cases where a significant void is present at the talus and/or ankle joint. The Silverback™ Span Plating System currently includes eight unique plates allowing for various approaches.
All Span Plates are anatomically contoured and are available in standard and long lengths with varying span distances to accommodate different allograft or cage heights required to fill the bone void. A proximal compression slot built into all plates may be used to compress the bone to graft interface. All plates have a transitional thickness to help evenly distribute force across the construct and to help guard against stress shielding during healing. Monster® Ø7.0 mm Screws accompany the system to aid in stabilizing the allograft prior to plate placement.
Mark Myerson, MD, a Silverback™ designing surgeon, said, “The Silverback™ Span Plates for an anterior or posterior approach to a complex ankle and hindfoot arthrodesis with bone loss really “bridge the gap”. These spanning plates have been designed to be used with structural allografts or cages where intramedullary fixation or a standard plate and screw construct is not feasible. These are unique, anatomic plates for all types of deformities associated with bone loss and allow for tremendous, stable fixation in conjunction with crossing tibiocalcaneal screws.”
Matt Jarboe, Paragon 28’s Chief Commercial Officer commented, “we are excited for this second addition to the Silverback™ Plating System in the past month, now providing surgeons with not only the first ankle fusion plates designed to straddle a nail, but now also the first plates designed to span a structural graft placed in the ankle joint as well.”
The addition of the Silverback™ Span Plating System bolsters Paragon 28’s Precision Ankle Solutions product offering, which includes the Gorilla® Ankle Fracture Plating System, APEX 3D™ Total Ankle Replacement, Phantom® TTC/TC Nail System, and Phantom® ActivCore™ Nail System. With this comprehensive portfolio, Paragon 28® provides its customers innovative ankle solutions for trauma, arthritis, and limb salvage.
Paragon 28® is grateful for the significant contributions that Paul Fortin, MD, Byron Hutchinson, DPM, Todd Irwin, MD, and Mark Myerson, MD, made in the design of this system.